Search for drugs:
Typing the drug name to query
BELINOSTAT
DIR Classification
Classification:Ambiguous
Severity Score:1.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- ADVERSE REACTIONS
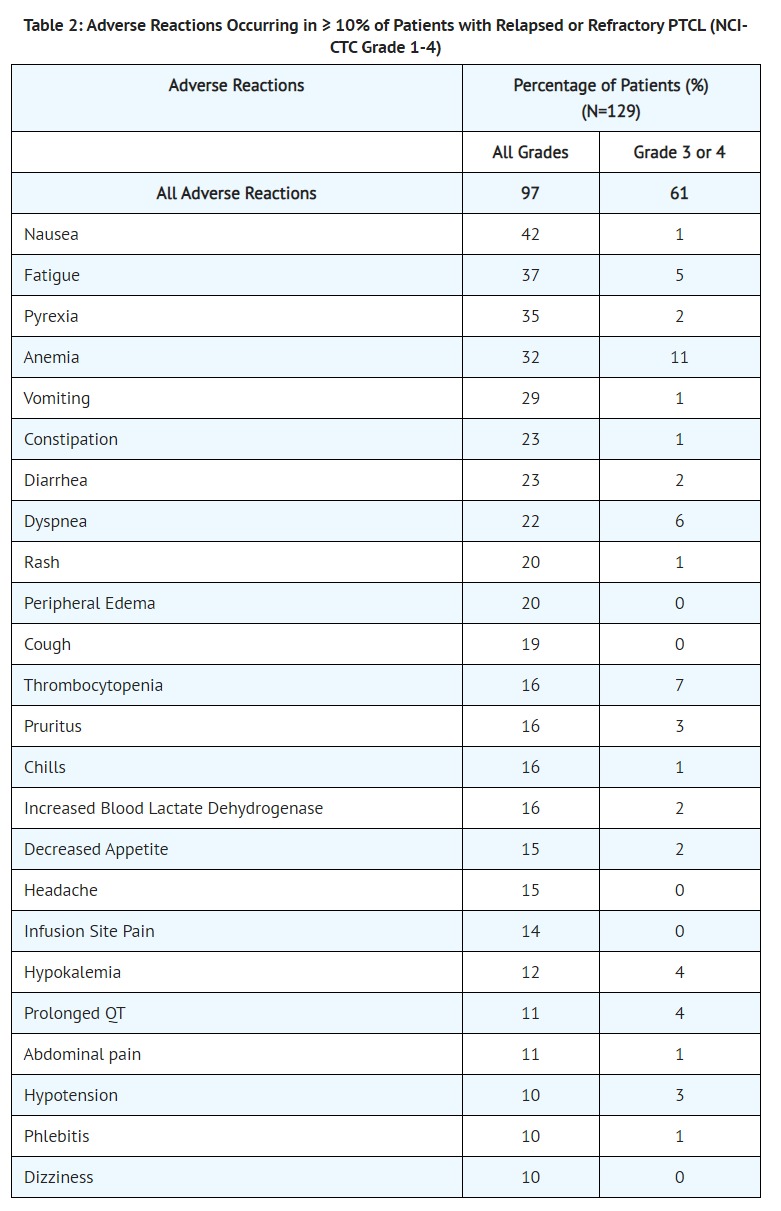
- [Serious Adverse Reactions]
- Sixty-one patients (47.3%) experienced serious adverse reactions while taking Beleodaq or within 30 days after their last dose of Beleodaq. The most common serious adverse reactions (> 2%) were pneumonia, pyrexia, infection, anemia, increased creatinine, thrombocytopenia, and multi-organ failure. One treatment-related death associated with hepatic failure was reported in the trial.
- One patient with baseline hyperuricemia and bulky disease experienced Grade 4 tumor lysis syndrome during the first cycle of treatment and died due to multi-organ failure. A treatment-related death from ventricular fibrillation was reported in another monotherapy clinical trial with Beleodaq. ECG analysis did not identify QTc prolongation.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
0
24092
Other ADRs
164
38381423
Odds Ratio = 0.0
Drug Property Information
ATC Code(s):
- L01XH04 - belinostat
- L01XH -
- L01X - OTHER ANTINEOPLASTIC AGENTS
- L01 - ANTINEOPLASTIC AGENTS
- L - ANTINEOPLASTIC AND IMMUNOMODULATING AGENTS
Active Ingredient:BELINOSTAT
Active Ingredient UNII:F4H96P17NZ
Drugbank ID:DB05015
PubChem Compound:6918638
CTD ID: C487081
CAS Number:866323-14-0
Dosage Form(s):injection, powder, lyophilized, for solution
Route(s) Of Administrator:intravenous
Daily Dose:
Chemical Structure: 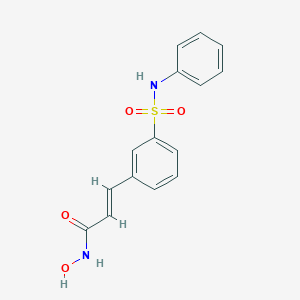

SMILE Code:
ONC(=O)\C=C\C1=CC=CC(=C1)S(=O)(=O)NC1=CC=CC=C1
ONC(=O)\C=C\C1=CC=CC(=C1)S(=O)(=O)NC1=CC=CC=C1
Reference
1: Phase 1 study of belinostat (PXD-101) and bortezomib (Velcade, PS-341) in patients with relapsed or refractory acute leukemia and myelodysplastic syndrome.
[Holkova Beata,Shafer Danielle,Yazbeck Victor,Dave Sandeep,Bose Prithviraj,Tombes Mary Beth,Shrader Ellen,Wan Wen,Bandyopadhyay Dipankar,Weir Caryn,Collins Elizabeth B,Garnett Amanda,Kmieciak Maciej,Roberts John D,Garcia-Manero Guillermo,Grant Steven]Leuk Lymphoma,2021 May;62(5):1187-1194. PMID: 33356689
2: Phase II study of the histone deacetylase inhibitor belinostat (PXD101) for the treatment of myelodysplastic syndrome (MDS).
[Cashen Amanda,Juckett Mark,Jumonville Alcee,Litzow Mark,Flynn P J,Eckardt John,LaPlant Betsy,Laumann Kristina,Erlichman Charles,DiPersio John]Ann Hematol,2012 Jan;91(1):33-8. PMID: 21538061
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.