Search for drugs:
Typing the drug name to query
CRIZOTINIB
DIR Classification
Classification:Moderate-DIQT concern
Severity Score:3.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- WARNINGS AND PRECAUTIONS
- QT Interval Prolongation
- QTc prolongation can occur in patients treated with XALKORI. Across clinical trials, 2.1% of 1616 patients had QTcF (corrected QT for heart rate by the Fridericia method) greater than or equal to 500 ms and 5% of 1582 patients had an increase from baseline QTcF greater than or equal to 60 ms by automated machine-read evaluation of ECGs.
- Avoid use of XALKORI in patients with congenital long QT syndrome. Monitor ECGs and electrolytes in patients with congestive heart failure, bradyarrhythmias, electrolyte abnormalities, or who are taking medications that are known to prolong the QT interval. Withhold, reduce dose, or permanently discontinue XALKORI for QT/QTc interval prolongation as recommended [see DOSAGE AND ADMINISTRATION (2.3), CLINICAL PHARMACOLOGY (12.2)].
- DRUG INTERACTIONS
- Drugs That Prolong the QT Interval
- XALKORI can prolong the QT/QTc interval. Avoid concomitant use of XALKORI with drugs that prolong the QT interval [see WARNINGS AND PRECAUTIONS (5.3), CLINICAL PHARMACOLOGY (12.2)].
- DOSAGE AND ADMINISTRATION
- ADVERSE REACTIONS
- The following clinically significant adverse reactions are described elsewhere in the labeling:
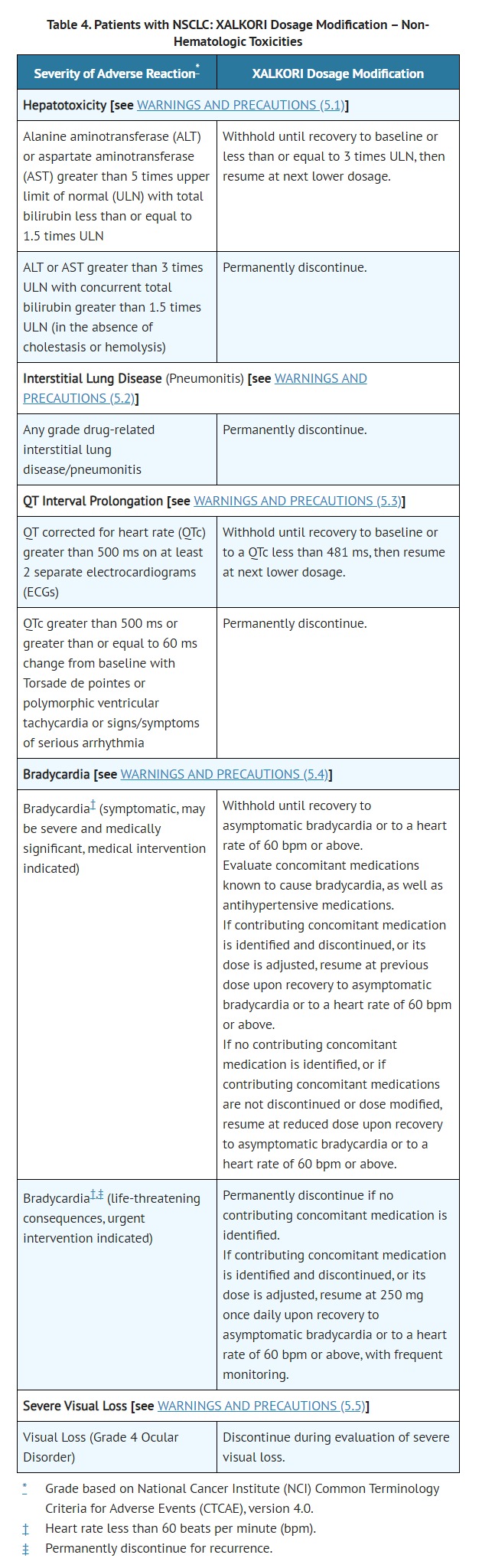
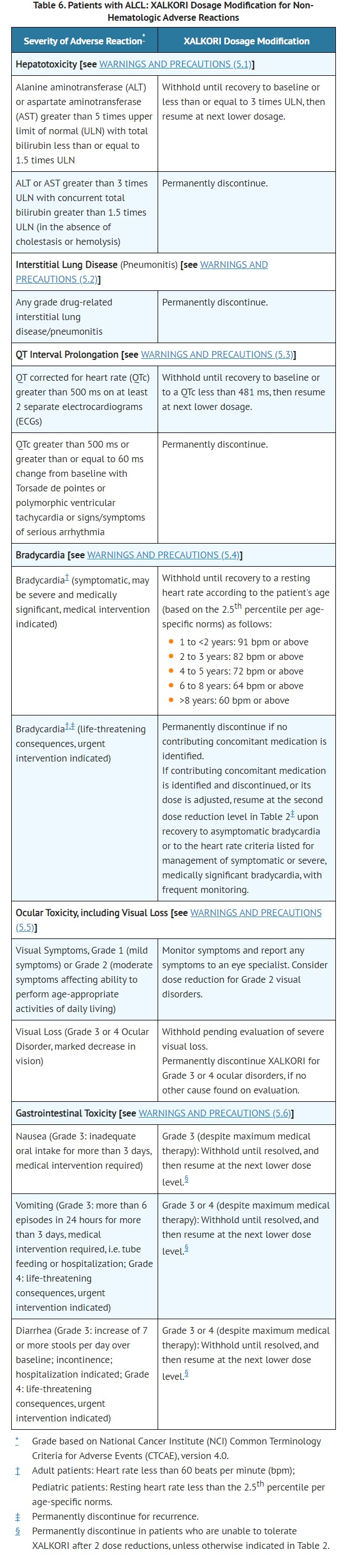
- Hepatotoxicity [see WARNINGS AND PRECAUTIONS (5.1)]
- Interstitial Lung Disease/Pneumonitis [see WARNINGS AND PRECAUTIONS (5.2)]
- QT Interval Prolongation [see WARNINGS AND PRECAUTIONS (5.3)]
- Bradycardia [see WARNINGS AND PRECAUTIONS (5.4)]
- Severe Visual Loss [see WARNINGS AND PRECAUTIONS (5.5)]
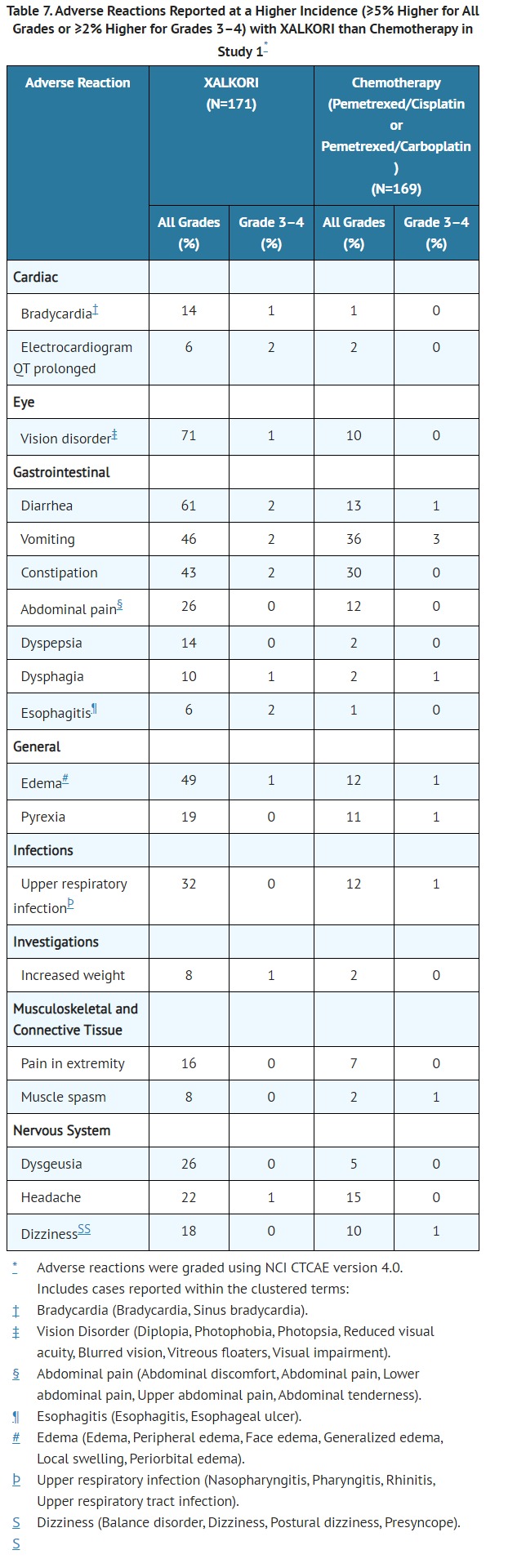
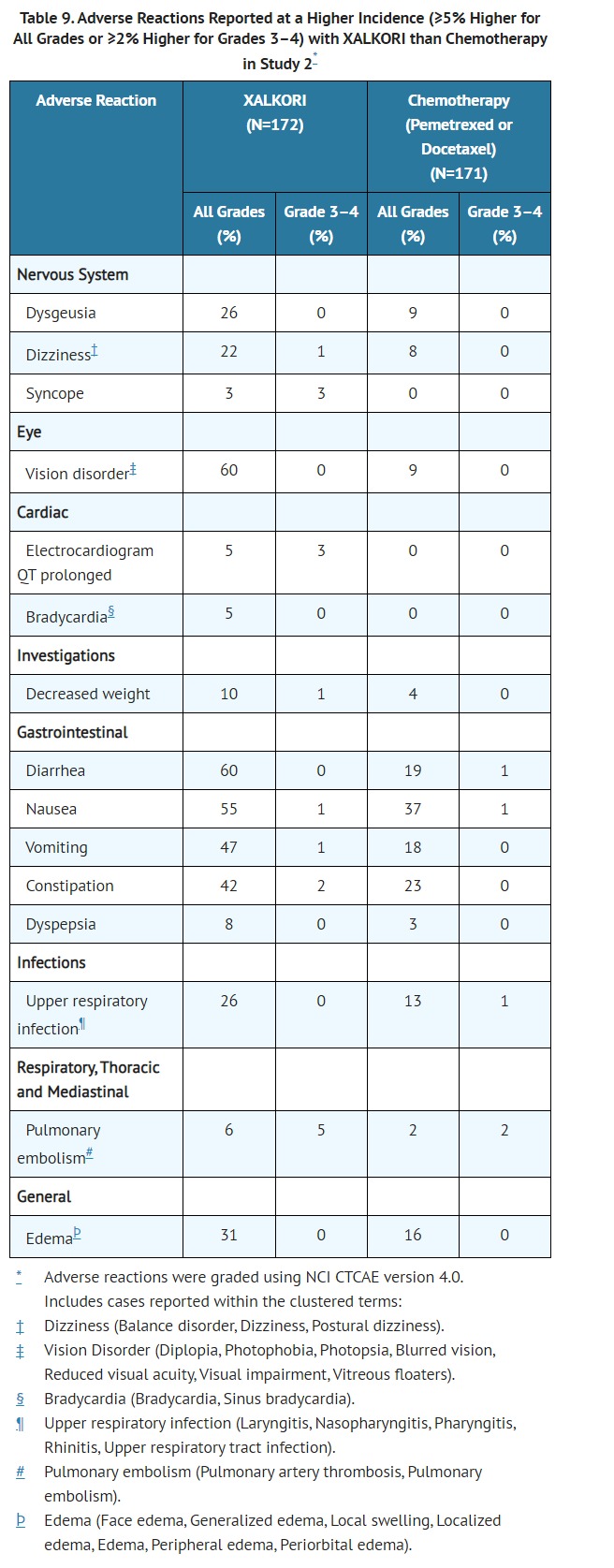
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Cardiac Electrophysiology
- In an ECG substudy conducted in 52 patients with ALK-positive NSCLC, the maximum mean QTcF (corrected QT by the Fridericia method) change from baseline was 12.3 ms (2-sided 90% upper CI: 19.5 ms) following administration of XALKORI 250 mg orally twice daily. An exposure-QT analysis suggested a crizotinib plasma concentration-dependent increase in QTcF [see WARNINGS AND PRECAUTIONS (5.3)].
- PATIENT PACKAGE INSERT
- What should I tell my healthcare provider before taking XALKORI?
- Before you take XALKORI, tell your healthcare provider if you:
- have heart problems, including a condition called long QT syndrome
- have liver or kidney problems
- have vision or eye problems
- have any other medical conditions
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
59
24033
Other ADRs
22819
38358768
Odds Ratio = 4.127
Drug Property Information
ATC Code(s):
- L01ED01 - crizotinib
- L01ED -
- L01E -
- L01 - ANTINEOPLASTIC AGENTS
- L - ANTINEOPLASTIC AND IMMUNOMODULATING AGENTS
Active Ingredient:CRIZOTINIB
Active Ingredient UNII:53AH36668S
Drugbank ID:DB08865
PubChem Compound:11626560
CTD ID:D000077547
PharmGKB:PA165946122
CAS Number:877399-52-5
Dosage Form(s):capsule
Route(s) Of Administrator:oral
Daily Dose:
Chemical Structure: 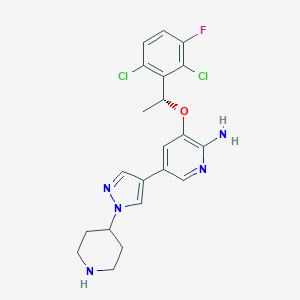

SMILE Code:
C[C@@H](OC1=CC(=CN=C1N)C1=CN(N=C1)C1CCNCC1)C1=C(Cl)C=CC(F)=C1Cl
C[C@@H](OC1=CC(=CN=C1N)C1=CN(N=C1)C1CCNCC1)C1=C(Cl)C=CC(F)=C1Cl
Reference
1: Dual Blockade of c-MET and the Androgen Receptor in Metastatic Castration-resistant Prostate Cancer: A Phase I Study of Concurrent Enzalutamide and Crizotinib.
[Tripathi Abhishek,Supko Jeffrey G,Gray Kathryn P,Melnick Zachary J,Regan Meredith M,Taplin Mary-Ellen,Choudhury Atish D,Pomerantz Mark M,Bellmunt Joaquim,Yu Channing,Sun Zijie,Srinivas Sandy,Kantoff Philip W,Sweeney Christopher J,Harshman Lauren C]Clin Cancer Res,2020 Dec 1;26(23):6122-6131. PMID: 32943461
2: Effect of the patient information brochure in communicating the risks associated with crizotinib treatment to patients with non-small cell lung cancer (NSCLC) in Europe.
[Huang Kui,Madison Terri,Wehler Beatrice,Tiseo Marcello,Wilner Keith D,Mo Jingping]Pharmacol Res Perspect,2020 Apr;8(2):e00570. PMID: 32232958
3: Crizotinib.
[Heigener David F,Reck Martin]Recent Results Cancer Res,2018;211:57-65. PMID: 30069759
4: Crizotinib-induced simultaneous multiple cardiac toxicities.
[Oyakawa Takuya,Muraoka Nao,Iida Kei,Kusuhara Masatoshi,Kawamura Takahisa,Naito Tateaki,Takahashi Toshiaki]Invest New Drugs,2018 Oct;36(5):949-951. PMID: 29717400
5: Crizotinib in patients with advanced, inoperable inflammatory myofibroblastic tumours with and without anaplastic lymphoma kinase gene alterations (European Organisation for Research and Treatment of Cancer 90101 CREATE): a multicentre, single-drug, prospective, non-randomised phase 2 trial.
[Schöffski Patrick,Sufliarsky Jozef,Gelderblom Hans,Blay Jean-Yves,Strauss Sandra J,Stacchiotti Silvia,Rutkowski Piotr,Lindner Lars H,Leahy Michael G,Italiano Antoine,Isambert Nicolas,Debiec-Rychter Maria,Sciot Raf,Van Cann Thomas,Marréaud Sandrine,Nzokirantevye Axelle,Collette Sandra,Wozniak Agnieszka]Lancet Respir Med,2018 Jun;6(6):431-441. PMID: 29669701
6: Current perspective: Osimertinib-induced QT prolongation: new drugs with new side-effects need careful patient monitoring.
[Schiefer Mart,Hendriks Lizza E L,Dinh Trang,Lalji Ulrich,Dingemans Anne-Marie C]Eur J Cancer,2018 Mar;91:92-98. PMID: 29413968
7: Effect of alectinib on cardiac electrophysiology: results from intensive electrocardiogram monitoring from the pivotal phase II NP28761 and NP28673 studies.
[Morcos Peter N,Bogman Katrijn,Hubeaux Stanislas,Sturm-Pellanda Carolina,Ruf Thorsten,Bordogna Walter,Golding Sophie,Zeaiter Ali,Abt Markus,Balas Bogdana]Cancer Chemother Pharmacol,2017 Mar;79(3):559-568. PMID: 28243683
8: Liposomes ameliorate Crizotinib- and Nilotinib-induced inhibition of the cardiac IKr channel and QTc prolongation.
[Shopp George M,Helson Lawrence,Bouchard Annie,Salvail Dany,Majeed Muhammad]Anticancer Res,2014 Sep;34(9):4733-40. PMID: 25202051
9: Crizotinib.
[Heigener David F,Reck Martin]Recent Results Cancer Res,2014;201:197-205. PMID: 24756793
10: Crizotinib.
Prescrire Int,2013 Nov;22(143):264. PMID: 24427836
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.