Search for drugs:
Typing the drug name to query
GLYCOPYRROLATE
DIR Classification
Classification:Ambiguous
Severity Score:1.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- ADVERSE REACTIONS
- In addition, the following adverse events have been reported from post-marketing experience with glycopyrrolate: malignant hyperthermia; cardiac arrhythmias (including bradycardia, ventricular tachycardia, ventricular fibrillation); cardiac arrest; hypertension; hypotension; seizures; and respiratory arrest. Post-marketing reports have included cases of heart block and QTc interval prolongation associated with the combined use of glycopyrrolate and an anticholinesterase. Injection site reactions including pruritus, edema, erythema, and pain have also been reported.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
0
24092
Other ADRs
0
38381587
Odds Ratio = N/A
Drug Property Information
ATC Code(s):
Active Ingredient:GLYCOPYRROLATE
Active Ingredient UNII:V92SO9WP2I
Drugbank ID:DB00986
PubChem Compound:9933193
CTD ID:D006024
PharmGKB:PA164754882
CAS Number:740028-90-4
Dosage Form(s):injection
Route(s) Of Administrator:intramuscular; intravenous
Daily Dose:
Chemical Structure: 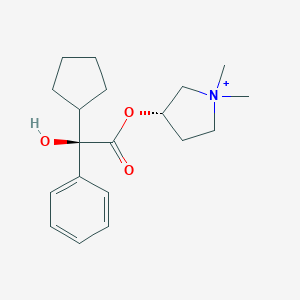

SMILE Code:
C[N+]1(C)CCC(C1)OC(=O)C(O)(C1CCCC1)C1=CC=CC=C1
C[N+]1(C)CCC(C1)OC(=O)C(O)(C1CCCC1)C1=CC=CC=C1
Reference
1: Cardiovascular safety profile of a fixed-dose combination of glycopyrrolate and formoterol fumarate delivered via metered dose inhaler using co-suspension delivery technology.
[Ferguson Gary T,Reisner Colin,Pearle James,DePetrillo Paolo,Maes Andrea,Martin Ubaldo J]Pulm Pharmacol Ther,2018 Apr;49:67-74. PMID: 29567116
2: A case report of QT prolongation with glycopyrronium bromide in a patient with chronic tamoxifen use.
[Chiu Michael H,Al-Majed Nawaf S,Stubbins Ryan,Pollmann Dylan,Sandhu Roopinder K]BMC Res Notes,2016 Jun 14;9:310. PMID: 27301406
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.