Search for drugs:
Typing the drug name to query
RITONAVIR
DIR Classification
Classification:Moderate-DIQT concern
Severity Score:3.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- DRUG INTERACTIONS
- Established and Other Potentially Significant Drug Interactions
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Cardiac Electrophysiology
- QTcF interval was evaluated in a randomized, placebo and active (moxifloxacin 400 mg once-daily) controlled crossover study in 45 healthy adults, with 10 measurements over 12 hours on Day 3. The maximum mean (95% upper confidence bound) time-matched difference in QTcF from placebo after baseline correction was 5.5 (7.6) milliseconds (msec) for 400 mg twice-daily ritonavir. Ritonavir 400 mg twice daily resulted in Day 3 ritonavir exposure that was approximately 1.5 fold higher than observed with ritonavir 600 mg twice-daily dose at steady state.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
19
24073
Other ADRs
17653
38363934
Odds Ratio = 1.716
Drug Property Information
ATC Code(s):
- J05AE03 - ritonavir
- J05AE - Protease inhibitors
- J05A - DIRECT ACTING ANTIVIRALS
- J05 - ANTIVIRALS FOR SYSTEMIC USE
- J - ANTIINFECTIVES FOR SYSTEMIC USE
Active Ingredient:RITONAVIR
Active Ingredient UNII:O3J8G9O825
Drugbank ID:DB00503
PubChem Compound:392622
CTD ID:D019438
PharmGKB:PA451260
CAS Number:155213-67-5
Dosage Form(s):powder; solution; tablet, film coated
Route(s) Of Administrator:oral
Daily Dose:
- 1200.0 mg/day J05AE03
Chemical Structure: 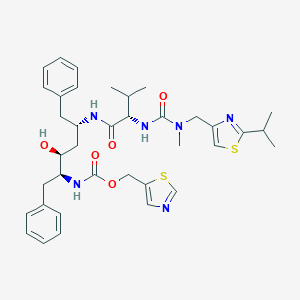

SMILE Code:
CC(C)[C@H](NC(=O)N(C)CC1=CSC(=N1)C(C)C)C(=O)N[C@H](C[C@H](O)[C@H](CC1=CC=CC=C1)NC(=O)OCC1=CN=CS1)CC1=CC=CC=C1
CC(C)[C@H](NC(=O)N(C)CC1=CSC(=N1)C(C)C)C(=O)N[C@H](C[C@H](O)[C@H](CC1=CC=CC=C1)NC(=O)OCC1=CN=CS1)CC1=CC=CC=C1
Reference
1: Effectiveness and cardiac safety of bedaquiline-based therapy for drug-resistant tuberculosis: a prospective cohort study.
[Brust James C M,Gandhi Neel R,Wasserman Sean,Maartens Gary,Omar Shaheed V,Ismail Nazir A,Campbell Angela,Joseph Lindsay,Hahn Alexandria,Allana Salim,Hernandez-Romieu Alfonso C,Zhang Chenshu,Mlisana Koleka,Viljoen Charle A,Zalta Benjamin,Ebrahim Ismaeel,Franczek Meghan,Master Iqbal,Ramangoaela Limpho,Te Riele Julian,Meintjes Graeme,PROBeX Study Team]Clin Infect Dis,2021 Apr 21;ciab335. PMID: 33882121
2: QTc prolongation in COVID-19 patients treated with hydroxychloroquine, chloroquine, azithromycin, or lopinavir/ritonavir: A systematic review and meta-analysis.
[Diaz-Arocutipa Carlos,Brañez-Condorena Ana,Hernandez Adrian V]Pharmacoepidemiol Drug Saf,2021 Jun;30(6):694-706. PMID: 33772933
3: Effect of COVID-19 medications on corrected QT interval and induction of torsade de pointes: Results of a multicenter national survey.
[Haghjoo Majid,Golipra Reza,Kheirkhah Jalal,Golabchi Allahyar,Shahabi Javad,Oni-Heris Saeed,Sami Ramin,Tajmirriahi Marzieh,Saravi Mehrdad,Khatami Mozhdeh,Varnasseri Mehran,Kiarsi Mohammadreza,Hejazi Seyed Fakhreddin,Yousefzadeh Rahaghi Mojtaba,Taherkhani Maryam,Ashraf Haleh,Keshmiri Mohammad Sadegh,Akbarzadeh Mohammad Ali,Bozorgi Ali,Mottaghizadeh Fateme,Hedayat Behnam,Heidarali Mona,Hajhossein Talasaz Azita]Int J Clin Pract,2021 Mar 24;e14182. PMID: 33759318
4: Smartphone electrocardiogram for QT interval monitoring in Coronavirus Disease 2019 (COVID-19) patients treated with Hydroxychloroquine.
[Andy Ko T Y,Chen L S,Pang I X,Ling H S,Wong T C,Sia Tonnii L L,Koh K T]Med J Malaysia,2021 Mar;76(2):125-130. PMID: 33742617
5: Pharmacotherapy Management for COVID-19 and Cardiac Safety: A Data Mining Approach for Pharmacovigilance Evidence from the FDA Adverse Event Reporting System (FAERS).
[Yuan Jing,Li Minghui,Yu Yiqun,Lee Tai-Ying,Lv Gang,Han Bing,Xiang Xiaoqiang,Lu Z Kevin]Drugs Real World Outcomes,2021 Feb 10;1-10. PMID: 33569736
6: Drug-drug interactions with candidate medications used for COVID-19 treatment: An overview.
[Rezaee Haleh,Pourkarim Fariba,Pourtaghi-Anvarian Samira,Entezari-Maleki Taher,Asvadi-Kermani Touraj,Nouri-Vaskeh Masoud]Pharmacol Res Perspect,2021 Feb;9(1):e00705. PMID: 33421347
7: Drug-drug interactions between COVID-19 treatments and antipsychotics drugs: integrated evidence from 4 databases and a systematic review.
[Plasencia-García Beatriz Oda,Rodríguez-Menéndez Gonzalo,Rico-Rangel María Isabel,Rubio-García Ana,Torelló-Iserte Jaime,Crespo-Facorro Benedicto]Psychopharmacology (Berl),2021 Feb;238(2):329-340. PMID: 33410987
8: QT Interval Monitoring and Drugs Management During COVID-19 Pandemic.
[Gasperetti Alessio,Schiavone Marco,Tondo Claudio,Mitacchione Gianfranco,Viecca Maurizio,Galli Massimo,Sarzi-Puttini Piercarlo,Forleo Giovanni Battista]Curr Clin Pharmacol,2020 Dec 24. PMID: 33357185
9: Global Safety Database Summary of COVID-19-Related Drug Utilization-Safety Surveillance: A Sponsor's Perspective.
[Beyzarov Elena,Chen Yan,Julg Rob,Naim Karen,Shah Jigna,Gregory William W,Ayoub Ayman,Caubel Patrick]Drug Saf,2021 Jan;44(1):95-105. PMID: 33354753
10: Off-label use of chloroquine, hydroxychloroquine, azithromycin and lopinavir/ritonavir in COVID-19 risks prolonging the QT interval by targeting the hERG channel.
[Zequn Zheng,Yujia Wu,Dingding Qian,Jiangfang Lian]Eur J Pharmacol,2021 Feb 15;893:173813. PMID: 33345848
11: Effect of hydroxychloroquine, azithromycin and lopinavir/ritonavir on the QT corrected interval in patients with COVID-19.
[Echarte-Morales Julio,Minguito-Carazo Carlos,Del Castillo-García Samuel,Borrego-Rodríguez Javier,Rodríguez-Santamarta Miguel,Sánchez-Muñoz Enrique,Bergel-García Rubén,González-Maniega Clea,Prieto-González Silvia,Menéndez-Suarez Paula,Tundidor-Sanz Elena,Benito-González Tomás,Fernández-Vázquez Felipe]J Electrocardiol,Jan-Feb 2021;64:30-35. PMID: 33307378
12: Potential Drug Interactions of Repurposed COVID-19 Drugs with Lung Cancer Pharmacotherapies.
[Baburaj Gayathri,Thomas Levin,Rao Mahadev]Arch Med Res,2021 Apr;52(3):261-269. PMID: 33257051
13: Investigational Treatments for COVID-19 may Increase Ventricular Arrhythmia Risk Through Drug Interactions.
[Varshneya Meera,Irurzun-Arana Itziar,Campana Chiara,Dariolli Rafael,Gutierrez Amy,Pullinger Taylor K,Sobie Eric A]CPT Pharmacometrics Syst Pharmacol,2021 Feb;10(2):100-107. PMID: 33205613
14: Effect of Triple Combination Therapy With Lopinavir-Ritonavir, Azithromycin, and Hydroxychloroquine on QT Interval and Arrhythmic Risk in Hospitalized COVID-19 Patients.
[Russo Vincenzo,Carbone Andreina,Mottola Filiberto Fausto,Mocerino Rosa,Verde Raffaele,Attena Emilio,Verde Nicoletta,Di Micco Pierpaolo,Nunziata Luigi,Santelli Francesco,Nigro Gerardo,Severino Sergio]Front Pharmacol,2020 Oct 8;11:582348. PMID: 33132915
15: Safety and efficacy of antiviral combination therapy in symptomatic patients of Covid-19 infection - a randomised controlled trial (SEV-COVID Trial): A structured summary of a study protocol for a randomized controlled trial.
[Panda Prasan Kumar,Bandyopadhyay Arkapal,Singh Budha Charan,Moirangthem Bikram,Chikara Gaurav,Saha Sarama,Bahurupi Yogesh Arvind]Trials,2020 Oct 20;21(1):866. PMID: 33081849
16: Identification of Drug-Induced Multichannel Block and Proarrhythmic Risk in Humans Using Continuous T Vector Velocity Effect Profiles Derived From Surface Electrocardiograms.
[Bystricky Werner,Maier Christoph,Gintant Gary,Bergau Dennis,Carter David]Front Physiol,2020 Sep 18;11:567383. PMID: 33071822
17: QT interval measurement with portable device during COVID-19 outbreak.
[González Nerea Torres,Acosta Luis Álvarez,Miranda Diego Valdivia,Plasencia Alejandro Iriarte,Cáceres Virginia Barreto,Zambrano Marx Rivera,Afonso Julio Salvador Hernández]Int J Cardiol Heart Vasc,2020 Oct;30:100644. PMID: 32964098
18: Saudi Heart Rhythm Society Task Force on Management of Potential Arrhythmogenicity Associated with Pharmacotherapy for COVID-19.
[AlShoaibi Naeem A,Maghrabi Khadijah,Alanazi Haitham,Harbi Mousa Al,Alghamdi Saleh]Ann Saudi Med,Sep-Oct 2020;40(5):365-372. PMID: 32954790
19: Risk Assessment of Drug-Induced Long QT Syndrome for Some COVID-19 Repurposed Drugs.
[Michaud Veronique,Dow Pamela,Al Rihani Sweilem B,Deodhar Malavika,Arwood Meghan,Cicali Brian,Turgeon Jacques]Clin Transl Sci,2021 Jan;14(1):20-28. PMID: 32888379
20: Pharmacogenomics of COVID-19 therapies.
[Takahashi Takuto,Luzum Jasmine A,Nicol Melanie R,Jacobson Pamala A]NPJ Genom Med,2020 Aug 18;5:35. PMID: 32864162
21: Predictive factors for cardiac conduction abnormalities with hydroxychloroquine-containing combinations for COVID-19.
[Padilla Sergio,Telenti Guillermo,Guillén Lucía,García José A,García-Abellán Javier,Ding Carolina,Mora Antonia,García-Pachón Eduardo,Gutiérrez Félix,Masiá Mar,COVID19-Elx Group]Int J Antimicrob Agents,2020 Oct;56(4):106142. PMID: 32853675
22: QTc prolongation during antiviral therapy in two COVID-19 patients.
[Zhu Suyan,Wang Jian,Wang Yong,Chu Jinguo,Liu Yao,Chen Xueqin,Chen Xiaomin]J Clin Pharm Ther,2020 Oct;45(5):1190-1193. PMID: 32779770
23: Physiologically-Based Pharmacokinetic Modeling to Predict the Clinical Efficacy of the Coadministration of Lopinavir and Ritonavir against SARS-CoV-2.
[Thakur Aarzoo,Tan Shawn Pei Feng,Chan James Chun Yip]Clin Pharmacol Ther,2020 Dec;108(6):1176-1184. PMID: 32767755
24: Drug interactions: a review of the unseen danger of experimental COVID-19 therapies.
[Hodge Catherine,Marra Fiona,Marzolini Catia,Boyle Alison,Gibbons Sara,Siccardi Marco,Burger David,Back David,Khoo Saye]J Antimicrob Chemother,2020 Dec 1;75(12):3417-3424. PMID: 32750131
25: Coronavirus Disease 2019 (COVID-19) and Transplantation: Pharmacotherapeutic Management of Immunosuppression Regimen.
[Mirjalili Mahtabalsadat,Shafiekhani Mojtaba,Vazin Afsaneh]Ther Clin Risk Manag,2020 Jul 3;16:617-629. PMID: 32694915
26: The Australasian COVID-19 Trial (ASCOT) to assess clinical outcomes in hospitalised patients with SARS-CoV-2 infection (COVID-19) treated with lopinavir/ritonavir and/or hydroxychloroquine compared to standard of care: A structured summary of a study protocol for a randomised controlled trial.
[Denholm Justin T,Davis Joshua,Paterson David,Roberts Jason,Morpeth Susan,Snelling Thomas,Zentner Dominica,Rees Megan,O'Sullivan Matthew,Price David,Bowen Asha,Tong Steven Y C,ASCOT Investigator Group]Trials,2020 Jul 14;21(1):646. PMID: 32665040
27: Clinical guidance for navigating the QTc-prolonging and arrhythmogenic potential of pharmacotherapy during the COVID-19 pandemic.
[Carron Jennifer,Sharif Zain,Hussein Hafiz,Kennedy Mark,McAdam Brendan,Sheahan Richard]Ir J Med Sci,2021 Feb;190(1):403-409. PMID: 32627127
28: Cardiovascular disease management during the coronavirus disease 2019 pandemic.
[Lee Wen-Hsien,Chen Ying-Chih,Chen Szu-Chia,Chen Chang-Jen,Hsu Po-Chao,Tsai Wei-Chung,Chu Chun-Yuan,Lee Chee-Siong,Lin Tsung-Hsien,Voon Wen-Chol,Kuo Chao-Hung,Su Ho-Ming]Int J Med Sci,2020 May 29;17(10):1340-1344. PMID: 32624690
29: Emerging and experimental treatments for COVID-19 and drug interactions with psychotropic agents.
[Bishara Delia,Kalafatis Chris,Taylor David]Ther Adv Psychopharmacol,2020 Jun 22;10:2045125320935306. PMID: 32612804
30: Investigational treatments for COVID-19 may increase ventricular arrhythmia risk through drug interactions.
[Varshneya Meera,Irurzun-Arana Itziar,Campana Chiara,Dariolli Rafael,Gutierrez Amy,Pullinger Taylor K,Sobie Eric A]medRxiv,2020 May 26;2020.05.21.20109397. PMID: 32511528
31: Effectiveness of Interferon Beta 1a, compared to Interferon Beta 1b and the usual therapeutic regimen to treat adults with moderate to severe COVID-19: structured summary of a study protocol for a randomized controlled trial.
[Irvani Seyed Sina Naghibi,Golmohammadi Maryam,Pourhoseingholi Mohamad Amin,Shokouhi Shervin,Darazam Ilad Alavi]Trials,2020 Jun 3;21(1):473. PMID: 32493468
32: "Off-label" use of hydroxychloroquine, azithromycin, lopinavir-ritonavir and chloroquine in COVID-19: A survey of cardiac adverse drug reactions by the French Network of Pharmacovigilance Centers.
[Gérard Alexandre,Romani Serena,Fresse Audrey,Viard Delphine,Parassol Nadège,Granvuillemin Aurélie,Chouchana Laurent,Rocher Fanny,Drici Milou-Daniel,French Network of Pharmacovigilance Centers]Therapie,Jul-Aug 2020;75(4):371-379. PMID: 32418730
33: Early experience with remdesivir in SARS-CoV-2 pneumonia.
[Durante-Mangoni Emanuele,Andini Roberto,Bertolino Lorenzo,Mele Ferruccio,Florio Letizia Lucia,Murino Patrizia,Corcione Antonio,Zampino Rosa]Infection,2020 Oct;48(5):779-782. PMID: 32418190
34: A review of the safety of favipiravir - a potential treatment in the COVID-19 pandemic?
[Pilkington Victoria,Pepperrell Toby,Hill Andrew]J Virus Erad,2020 Apr 30;6(2):45-51. PMID: 32405421
35: Cardiac safety of off-label COVID-19 drug therapy: a review and proposed monitoring protocol.
[Naksuk Niyada,Lazar Sorin,Peeraphatdit Thoetchai Bee]Eur Heart J Acute Cardiovasc Care,2020 Apr;9(3):215-221. PMID: 32372695
36: [Medication and comedication in COVID-19 patients].
[Lenkens M,de Wit H,Danser A H,Esselink A C,Horikx A,Ten Oever J,van de Veerdonk F,Kramers C]Ned Tijdschr Geneeskd,2020 Mar 25;164:D4995. PMID: 32324352
37: Utilizing PBPK Modeling to Evaluate the Potential of a Significant Drug-Drug Interaction Between Clopidogrel and Dasabuvir: A Scientific Perspective.
[Arya V,Zhao P,Reynolds K S,Mishra P,Younis I R]Clin Pharmacol Ther,2017 Oct;102(4):578-580. PMID: 28444890
38: Potential interactions between HIV drugs, ritonavir and efavirenz and anticancer drug, nilotinib--a study in primary cultures of human hepatocytes that is applicable to HIV patients with cancer.
[Pillai Venkateswaran C,Parise Robert A,Christner Susan M,Rudek Michelle A,Beumer Jan H,Venkataramanan Raman]J Clin Pharmacol,2014 Nov;54(11):1272-9. PMID: 24846165
39: Atazanavir induced first degree atrioventricular block and ventricular tachycardia: a case report.
[Santimaleeworagun Wichai,Pattharachayakul Suthipom,Chusri Sarunyou,Chayagul Pantip]J Med Assoc Thai,2013 Apr;96(4):501-3. PMID: 23691707
40: Protease inhibitor-associated QT interval prolongation.
[Hunt Kimberley,Hughes Christine A,Hills-Nieminen Cara]Ann Pharmacother,2011 Dec;45(12):1544-50. PMID: 22128044
41: Thorough QT/QTc study of ritonavir-boosted saquinavir following multiple-dose administration of therapeutic and supratherapeutic doses in healthy participants.
[Zhang Xiaoping,Jordan Paul,Cristea Laura,Salgo Miklos,Farha Rana,Kolis Stanley,Lee Lois S]J Clin Pharmacol,2012 Apr;52(4):520-9. PMID: 21558456
42: Effect of vicriviroc on the QT/corrected QT interval and central nervous system in healthy subjects.
[O'Mara Edward,Kasserra Claudia,Huddlestone John Robert,Wan Yuntao,Soni Peter,Caceres Maria,Medlock Matthew,Morrison Royce,Devinsky Orrin]Antimicrob Agents Chemother,2010 Jun;54(6):2448-54. PMID: 20350942
43: Ritonavir 100 mg does not cause QTc prolongation in healthy subjects: a possible role as CYP3A inhibitor in thorough QTc studies.
[Sarapa N,Nickens D J,Raber S R,Reynolds R R,Amantea M A]Clin Pharmacol Ther,2008 Jan;83(1):153-9. PMID: 17581594
44: Methadone-induced Torsade de pointes after stopping lopinavir-ritonavir.
[Lüthi B,Huttner A,Speck R F,Mueller N J]Eur J Clin Microbiol Infect Dis,2007 May;26(5):367-9. PMID: 17440756
45: Mechanism-based inhibition of cytochrome P450 3A4 by therapeutic drugs.
[Zhou Shufeng,Yung Chan Sui,Cher Goh Boon,Chan Eli,Duan Wei,Huang Min,McLeod Howard L]Clin Pharmacokinet,2005;44(3):279-304. PMID: 15762770
46: Blockade of HERG channels by HIV protease inhibitors.
[Anson Blake D,Weaver Joel G R,Ackerman Michael J,Akinsete Omobosola,Henry Keith,January Craig T,Badley Andrew D]Lancet,2005 Feb 19-25;365(9460):682-6. PMID: 15721475
47: Inhibition of cytochrome P450 3A: relevant drug interactions in gastroenterology.
[Sagir A,Schmitt M,Dilger K,Häussinger D]Digestion,2003;68(1):41-8. PMID: 12949438
48: Drug interactions with cisapride: clinical implications.
[Michalets E L,Williams C R]Clin Pharmacokinet,2000 Jul;39(1):49-75. PMID: 10926350
49: Pharmacokinetic-pharmacodynamic consequences and clinical relevance of cytochrome P450 3A4 inhibition.
[Dresser G K,Spence J D,Bailey D G]Clin Pharmacokinet,2000 Jan;38(1):41-57. PMID: 10668858
50: Systemic antifungal agents. Drug interactions of clinical significance.
[Albengres E,Le Louët H,Tillement J P]Drug Saf,1998 Feb;18(2):83-97. PMID: 9512916
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.