Search for drugs:
Typing the drug name to query
TRAMADOL HYDROCHLORIDE
DIR Classification
Classification:Moderate-DIQT concern
Severity Score:2.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- OVERDOSAGE
- Clinical Presentation
- Acute overdosage with Tramadol Hydrochloride Extended-Release Capsules can be manifested by respiratory depression, somnolence progressing to stupor or coma, skeletal muscle flaccidity, cold and clammy skin, constricted pupils, and, in some cases, pulmonary edema, bradycardia, QT prolongation, hypotension, partial or complete airway obstruction, atypical snoring, and death. Marked mydriasis rather than miosis may be seen with hypoxia in overdose situations. [see Clinical Pharmacology (12.2)]
- ADVERSE REACTIONS
- Postmarketing Experience
- The following adverse reactions have been identified during post approval use of tramadol. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- QT prolongation/torsade de pointes: Cases of QT prolongation and/or torsade de pointes have been reported with tramadol use. Many of these cases were reported in patients taking another drug labeled for QT prolongation, in patients with a risk factor for QT prolongation (e.g., hypokalemia), or in overdose setting.
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- The effect of oral tramadol on the QTcF interval was evaluated in a double-blind, randomized, four-way crossover, placebo- and positive- (moxifloxacin) controlled study in 68 adult male and female healthy subjects. At a 600 mg/day dose (1.5-fold the maximum immediate-release daily dose), the study demonstrated no significant effect on the QTcF interval.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
100
23992
Other ADRs
76725
38304862
Odds Ratio = 2.081
Drug Property Information
ATC Code(s):
- N02AX02 - tramadol hydrochloride
- N02AX - Other opioids
- N02A - OPIOIDS
- N02 - ANALGESICS
- N - NERVOUS SYSTEM
Active Ingredient:TRAMADOL HYDROCHLORIDE
Active Ingredient UNII:9N7R477WCK
Drugbank ID:DB00193
PubChem Compound:33741
CTD ID:D014147
PharmGKB:PA451735
CAS Number:27203-92-5
Dosage Form(s):capsule, extended release
Route(s) Of Administrator:oral
Daily Dose:
- 300.0 mg/day N02AX02
Chemical Structure: 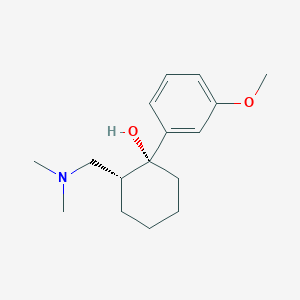

SMILE Code:
COC1=CC=CC(=C1)[C@@]1(O)CCCC[C@@H]1CN(C)C
COC1=CC=CC(=C1)[C@@]1(O)CCCC[C@@H]1CN(C)C
Reference
1: Tramadol Hydrochloride at Steady State Lacks Clinically Relevant QTc Interval Increases in Healthy Adults.
[Massarella Joseph,Ariyawansa Jay,Natarajan Jaya,Francke Stephan,Murtaugh Thomas,DeLemos Byron,Vaughan Subusola,Fonseca Sergio]Clin Pharmacol Drug Dev,2019 Jan;8(1):95-106. PMID: 29775246
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.