Search for drugs:
Typing the drug name to query
ENCORAFENIB
DIR Classification
Classification:Most-DIQT concern
Severity Score:4.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- WARNINGS AND PRECAUTIONS
- QT Prolongation
- BRAFTOVI is associated with dose-dependent QTc interval prolongation in some patients [see CLINICAL PHARMACOLOGY (12.2)]. In COLUMBUS, an increase in QTcF to > 500 ms was measured in 0.5% (1/192) of patients who received BRAFTOVI in combination with binimetinib.
- Monitor patients who already have or who are at significant risk of developing QTc prolongation, including patients with known long QT syndromes, clinically significant bradyarrhythmias, severe or uncontrolled heart failure and those taking other medicinal products associated with QT prolongation. Correct hypokalemia and hypomagnesemia prior to and during BRAFTOVI administration. Withhold, reduce dose, or permanently discontinue for QTc > 500 ms [see DOSAGE AND ADMINISTRATION (2.5), ADVERSE REACTIONS (6.1)].
- DRUG INTERACTIONS
- Drugs That Prolong the QT Interval
- BRAFTOVI is associated with dose-dependent QTc interval prolongation [see WARNINGS AND PRECAUTIONS (5.5), CLINICAL PHARMACOLOGY (12.2)]. Avoid coadministration of BRAFTOVI with drugs known to prolong the QT/QTc interval.
- DOSAGE AND ADMINISTRATION
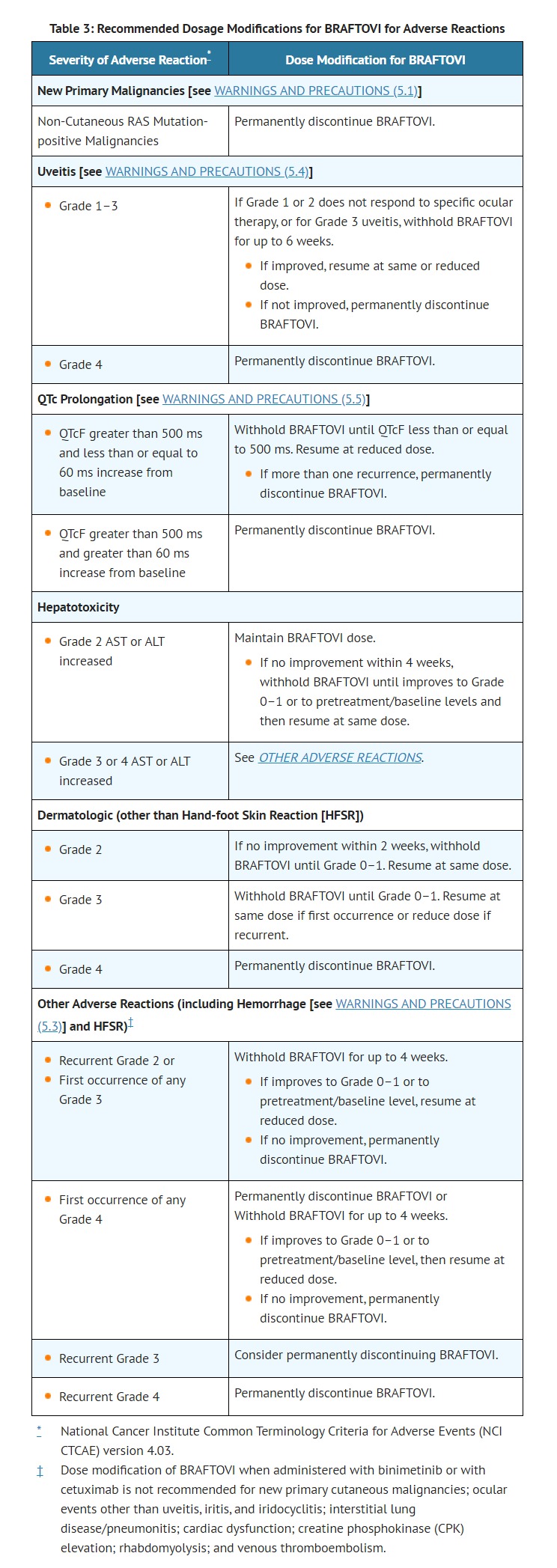
- ADVERSE REACTIONS
- The following adverse reactions are described elsewhere in the labeling:
- New Primary Malignancies [see WARNINGS AND PRECAUTIONS (5.1)]
- Hemorrhage [see WARNINGS AND PRECAUTIONS (5.3)]
- Uveitis [see WARNINGS AND PRECAUTIONS (5.4)]
- QT Prolongation [see WARNINGS AND PRECAUTIONS (5.5)]
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Cardiac Electrophysiology
- A dedicated study to evaluate the QT prolongation potential of BRAFTOVI has not been conducted. BRAFTOVI is associated with dose-dependent QTc interval prolongation. Based on a central tendency analysis of QTc in a study of adult patients with melanoma who received the recommended dose of BRAFTOVI in combination with binimetinib, the largest mean (90% CI) QTcF change from baseline (ΔQTcF) was 18 (14 to 22) ms [see WARNINGS AND PRECAUTIONS (5.5)].
- PATIENT COUNSELING INFORMATION
- QT Prolongation
- Advise patients that BRAFTOVI can cause QTc interval prolongation and to inform their physician if they have any QTc interval prolongation symptoms, such as syncope [see WARNINGS AND PRECAUTIONS (5.5)].
- What are the possible side effects of BRAFTOVI?
- BRAFTOVI may cause serious side effects, including:
- Changes in the electrical activity of your heart called QT prolongation. QT prolongation can cause irregular heartbeats that can be life threatening. Your healthcare provider should do tests before you start taking BRAFTOVI with binimetinib or cetuximab and during your treatment to check your body salts (electrolytes). Tell your healthcare provider right away if you feel faint, lightheaded, dizzy or if you feel your heart beating irregularly or fast while taking BRAFTOVI with binimetinib or cetuximab. These symptoms may be related to QT prolongation.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
4
24088
Other ADRs
1664
38379923
Odds Ratio = 3.831
Drug Property Information
ATC Code(s):
- L01EC03 - encorafenib
- L01EC -
- L01E -
- L01 - ANTINEOPLASTIC AGENTS
- L - ANTINEOPLASTIC AND IMMUNOMODULATING AGENTS
Active Ingredient:ENCORAFENIB
Active Ingredient UNII:8L7891MRB6
Drugbank ID:DB11718
PubChem Compound:50922675
CTD ID:C000601108
PharmGKB:PA166179872
CAS Number:1269440-17-6
Dosage Form(s):capsule
Route(s) Of Administrator:oral
Daily Dose:
Chemical Structure: 

SMILE Code:
COC(=O)N[C@@H](C)CNC1=NC=CC(=N1)C1=CN(N=C1C1=CC(Cl)=CC(NS(C)(=O)=O)=C1F)C(C)C
COC(=O)N[C@@H](C)CNC1=NC=CC(=N1)C1=CN(N=C1C1=CC(Cl)=CC(NS(C)(=O)=O)=C1F)C(C)C
Reference
1: Cardiovascular Adverse Events Associated With BRAF and MEK Inhibitors: A Systematic Review and Meta-analysis.
[Mincu Raluca I,Mahabadi Amir A,Michel Lars,Mrotzek Simone M,Schadendorf Dirk,Rassaf Tienush,Totzeck Matthias]JAMA Netw Open,2019 Aug 2;2(8):e198890. PMID: 31397860
2: Tolerability of BRAF/MEK inhibitor combinations: adverse event evaluation and management.
[Heinzerling Lucie,Eigentler Thomas K,Fluck Michael,Hassel Jessica C,Heller-Schenck Daniela,Leipe Jan,Pauschinger Matthias,Vogel Arndt,Zimmer Lisa,Gutzmer Ralf]ESMO Open,2019 May 23;4(3):e000491. PMID: 31231568
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.