Search for drugs:
Typing the drug name to query
LEVALBUTEROL TARTRATE
DIR Classification
Classification:Ambiguous
Severity Score:1.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- WARNINGS AND PRECAUTIONS
- Cardiovascular Effects
- XOPENEX HFA, like other beta-adrenergic agonists, can produce clinically significant cardiovascular effects in some patients, as measured by heart rate, blood pressure, and symptoms. Although such effects are uncommon after administration of XOPENEX HFA at recommended doses, if they occur, the drug may need to be discontinued. In addition, beta-agonists have been reported to produce electrocardiogram (ECG) changes, such as flattening of the T-wave, prolongation of the QTc interval, and ST segment depression. The clinical significance of these findings is unknown. Therefore, XOPENEX HFA, like all sympathomimetic amines, should be used with caution in patients with cardiovascular disorders, especially coronary insufficiency, cardiac arrhythmias, and hypertension.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
3
24089
Other ADRs
3005
38378582
Odds Ratio = 1.591
Drug Property Information
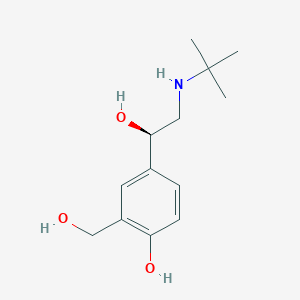
SMILE Code:
CC(C)(C)NC[C@H](O)C1=CC(CO)=C(O)C=C1
CC(C)(C)NC[C@H](O)C1=CC(CO)=C(O)C=C1
Reference
N/A
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.