Search for drugs:
Typing the drug name to query
FLUOXETINE HYDROCHLORIDE
DIR Classification
Classification:Most-DIQT concern
Severity Score:4.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- WARNINGS AND PRECAUTIONS
- QT Prolongation
- Post-marketing cases of QT interval prolongation and ventricular arrhythmia including Torsades de Pointes have been reported in patients treated with fluoxetine. Fluoxetine should be used with caution in patients with congenital long QT syndrome; a previous history of QT prolongation; a family history of long QT syndrome or sudden cardiac death; and other conditions that predispose to QT prolongation and ventricular arrhythmia. Such conditions include concomitant use of drugs that prolong the QT interval; hypokalemia or hypomagnesemia; recent myocardial infarction, uncompensated heart failure, bradyarrhythmias, and other significant arrhythmias; and conditions that predispose to increased fluoxetine exposure (overdose, hepatic impairment, use of CYP2D6 inhibitors, CYP2D6 poor metabolizer status, or use of other highly protein-bound drugs). Fluoxetine is primarily metabolized by CYP2D6
- Pimozide and thioridazine are contraindicated for use with fluoxetine. Avoid the concomitant use of drugs known to prolong the QT interval. These include specific antipsychotics (e.g., ziprasidone, iloperidone, chlorpromazine, mesoridazine, droperidol,); specific antibiotics (e.g., erythromycin, gatifloxacin, moxifloxacin, sparfloxacin); Class 1A antiarrhythmic medications (e.g., quinidine, procainamide); Class III antiarrhythmics (e.g., amiodarone, sotalol); and others (e.g., pentamidine, levomethadyl acetate, methadone, halofantrine, mefloquine, dolasetron mesylate, probucol or tacrolimus)
- Consider ECG assessment and periodic ECG monitoring if initiating treatment with fluoxetine in patients with risk factors for QT prolongation and ventricular arrhythmia. Consider discontinuing fluoxetine and obtaining a cardiac evaluation if patients develop signs or symptoms consistent with ventricular arrhythmia.
- DRUG INTERACTIONS
- Pimozide — Concomitant use in patients taking pimozide is contraindicated. Pimozide can prolong the QT interval. Fluoxetine can increase the level of pimozide through inhibition of CYP2D6. Fluoxetine can also prolong the QT interval. Clinical studies of pimozide with other antidepressants demonstrate an increase in drug interaction or QT prolongation. While a specific study with pimozide and fluoxetine has not been conducted, the potential for drug interactions or QT prolongation warrants restricting the concurrent use of pimozide and fluoxetine[see CONTRAINDICATIONS (4.2), WARNINGS AND PRECAUTIONS (5.11), and DRUG INTERACTIONS (7.8)] .
- Thioridazine — Thioridazine should not be administered with fluoxetine or within a minimum of 5 weeks after fluoxetine has been discontinued, because of the risk of QT Prolongation [see CONTRAINDICATIONS (4.2), WARNINGS AND PRECAUTIONS (5.11), and DRUG INTERACTIONS (7.8)] .
- Thioridazine administration produces a dose-related prolongation of the QT interval, which is associated with serious ventricular arrhythmias, such as Torsades de Pointes-type arrhythmias, and sudden death. This risk is expected to increase with fluoxetine-induced inhibition of thioridazine metabolism.
- [ Drugs that Prolong the QT Interval]
- Do not use fluoxetine in combination with thioridazine or pimozide. Use fluoxetine with caution in combination with other drugs that cause QT prolongation. These include: specific antipsychotics (e.g., ziprasidone, iloperidone, chlorpromazine, mesoridazine, droperidol); specific antibiotics (e.g., erythromycin, gatifloxacin, moxifloxacin, sparfloxacin); Class 1A antiarrhythmic medications (e.g., quinidine, procainamide); Class III antiarrhythmics (e.g., amiodarone, sotalol); and others (e.g., pentamidine, levomethadyl acetate, methadone, halofantrine, mefloquine, dolasetron mesylate, probucol or tacrolimus). Fluoxetine is primarily metabolized by CYP2D6. Concomitant treatment with CYP2D6 inhibitors can increase the concentration of fluoxetine. Concomitant use of other highly protein-bound drugs can increase the concentration of fluoxetine [see CONTRAINDICATIONS (4.2), WARNINGS AND PRECAUTIONS (5.11), DRUG INTERACTIONS (7.7), and CLINICAL PHARMACOLOGY (12.3)].
- CONTRAINDICATIONS
- Pimozide and thioridazine prolong the QT interval. Fluoxetine capsules can increase the levels of pimozide and thioridazine through inhibition of CYP2D6. Fluoxetine capsules can also prolong the QT interval.
- OVERDOSAGE
- Human Experience
- Other important adverse reactions reported with fluoxetine overdose (single or multiple drugs) include coma, delirium, ECG abnormalities (such as nodal rhythm, QT interval prolongation and ventricular arrhythmias, including Torsades de Pointes-type arrhythmias), hypotension, mania, neuroleptic malignant syndrome-like reactions, pyrexia, stupor, and syncope.
- ADVERSE REACTIONS
- QT Prolongation [see WARNINGS AND PRECAUTIONS (5.11)]
- Investigations — Frequent: QT interval prolongation (QT cF ≥450 msec) . QT prolongation data are based on routine ECG measurements in clinical trials.
- Voluntary reports of adverse reactions temporally associated with fluoxetine that have been received since market introduction and that may have no causal relationship with the drug include the following: aplastic anemia, atrial fibrillation 1, cataract, cerebrovascular accident 1, cholestatic jaundice, dyskinesia (including, for example, a case of buccal-lingual-masticatory syndrome with involuntary tongue protrusion reported to develop in a 77-year-old female after 5 weeks of fluoxetine therapy and which completely resolved over the next few months following drug discontinuation), eosinophilic pneumonia 1, epidermal necrolysis, erythema multiforme, erythema nodosum, exfoliative dermatitis, galactorrhea, gynecomastia, heart arrest 1, hepatic failure/necrosis, hyperprolactinemia, hypoglycemia, immune-related hemolytic anemia, kidney failure, memory impairment, movement disorders developing in patients with risk factors including drugs associated with such reactions and worsening of pre-existing movement disorders, optic neuritis, pancreatitis 1, pancytopenia, pulmonary embolism, pulmonary hypertension, QT prolongation, Stevens-Johnson syndrome, thrombocytopenia 1, thrombocytopenic purpura, ventricular tachycardia (including Torsades de Pointes–type arrhythmias), vaginal bleeding, and violent behaviors 1.
- PATIENT COUNSELING INFORMATION
- QT Prolongation
- Patients should be advised that QT interval prolongation and ventricular arrhythmia including Torsades de Pointes have been reported in patients treated with fluoxetine. Signs and symptoms of ventricular arrhythmia include fast, slow, or irregular heart rate, dyspnea, syncope, or dizziness, which may indicate serious cardiac arrhythmia
- [MEDICATION GUIDE]
- Changes in the electrical activity of your heart (QT prolongation and ventricular arrhythmia including Torsades de Pointes). This condition can be life threatening. The symptoms may include:
- fast, slow, or irregular heartbeat
- shortness of breath
- dizziness or fainting
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
341
23751
Other ADRs
75655
38305932
Odds Ratio = 7.27
Drug Property Information
ATC Code(s):
- N06AB03 - fluoxetine hydrochloride
- N06AB - Selective serotonin reuptake inhibitors
- N06A - ANTIDEPRESSANTS
- N06 - PSYCHOANALEPTICS
- N - NERVOUS SYSTEM
- D01AC15 - fluoxetine hydrochloride
- D01AC - Imidazole and triazole derivatives
- D01A - ANTIFUNGALS FOR TOPICAL USE
- D01 - ANTIFUNGALS FOR DERMATOLOGICAL USE
- D - DERMATOLOGICALS
Active Ingredient:FLUOXETINE HYDROCHLORIDE
Active Ingredient UNII:I9W7N6B1KJ
Drugbank ID:DB00472
PubChem Compound:3386
CTD ID:D005473
PharmGKB:PA449673
CAS Number:54910-89-3
Dosage Form(s):capsule
Route(s) Of Administrator:oral
Daily Dose:
- 20.0 mg/day N06AB03
Chemical Structure: 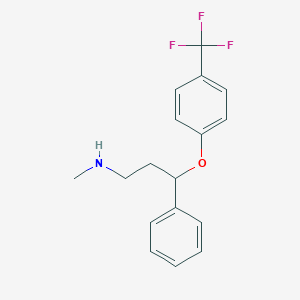

SMILE Code:
CNCCC(OC1=CC=C(C=C1)C(F)(F)F)C1=CC=CC=C1
CNCCC(OC1=CC=C(C=C1)C(F)(F)F)C1=CC=CC=C1
Reference
N/A
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.