Search for drugs:
Typing the drug name to query
GLASDEGIB
DIR Classification
Classification:Most-DIQT concern
Severity Score:4.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- WARNINGS AND PRECAUTIONS
- QTc Interval Prolongation
- Patients treated with DAURISMO can develop QTc prolongation and ventricular arrhythmias, including ventricular fibrillation and ventricular tachycardia. Of the 98 evaluable patients treated with DAURISMO 100 mg in combination with low-dose cytarabine in the clinical trial, 5% were found to have a QTc interval greater than 500 ms and 4% of patients had an increase from baseline QTc greater than 60 ms. The clinical trial excluded patients with baseline QTc of greater than 470 ms or with a history of long QT syndrome or uncontrolled cardiovascular disease.
- Monitor electrocardiograms (ECGs) and electrolytes [see DOSAGE AND ADMINISTRATION (2.2)]. Concomitant use of DAURISMO with drugs known to prolong the QTc interval and CYP3A4 inhibitors may increase the risk of QTc interval prolongation [see DRUG INTERACTIONS (7), CLINICAL PHARMACOLOGY (12.2)]. In patients with congenital long QT syndrome, congestive heart failure, electrolyte abnormalities, or those who are taking medications known to prolong the QTc interval, more frequent ECG monitoring is recommended.
- Interrupt DAURISMO if QTc increases to greater than 500 ms. Discontinue DAURISMO permanently for patients who develop QTc interval prolongation with signs or symptoms of life-threatening arrhythmia [see DOSAGE AND ADMINISTRATION (2.2)].
- DRUG INTERACTIONS
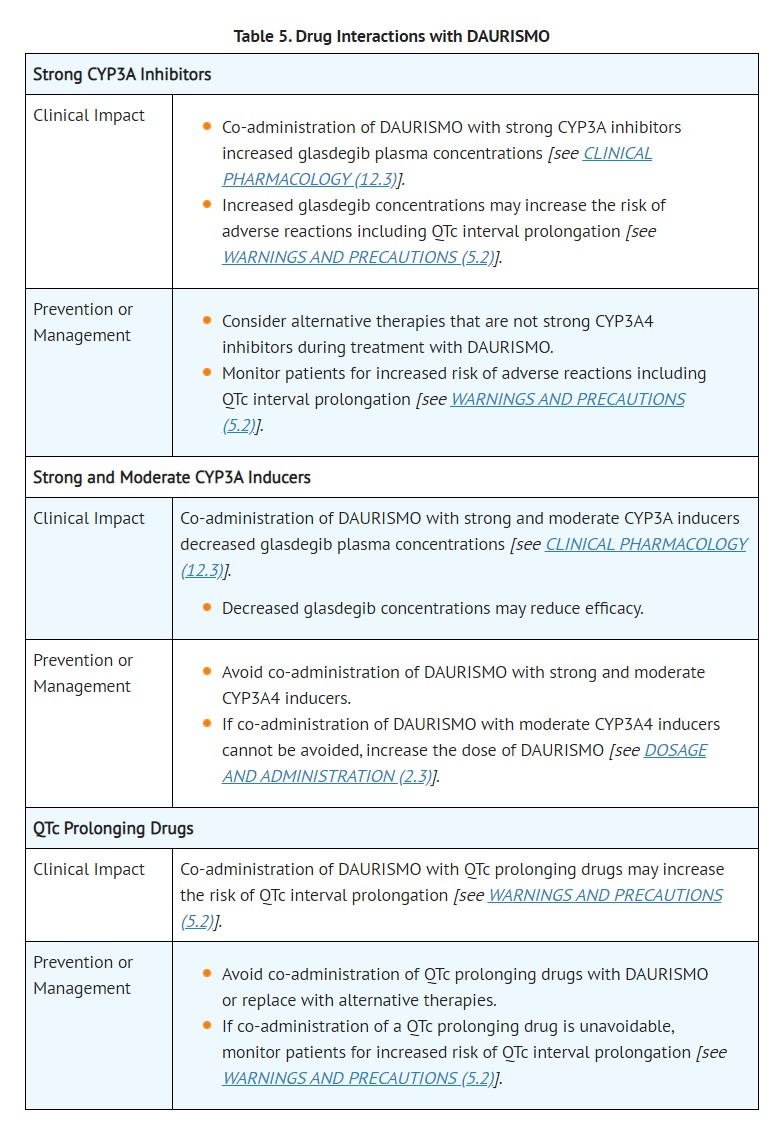
- DOSAGE AND ADMINISTRATION
- Monitoring and Dosage Modifications
- Assess complete blood counts, electrolytes, renal, and hepatic function prior to the initiation of DAURISMO and at least once weekly for the first month. Monitor electrolytes and renal function once monthly for the duration of therapy. Obtain serum creatine kinase levels prior to initiating DAURISMO and as indicated clinically thereafter (e.g., if muscle symptoms are reported). Monitor electrocardiograms (ECGs) prior to the initiation of DAURISMO, approximately one week after initiation, and then once monthly for the next two months to assess for QTc prolongation. Repeat ECG if abnormal. Certain patients may require more frequent and ongoing ECG monitoring [see WARNINGS AND PRECAUTIONS (5.2)]. Manage any abnormalities promptly [see ADVERSE REACTIONS (6.1)].
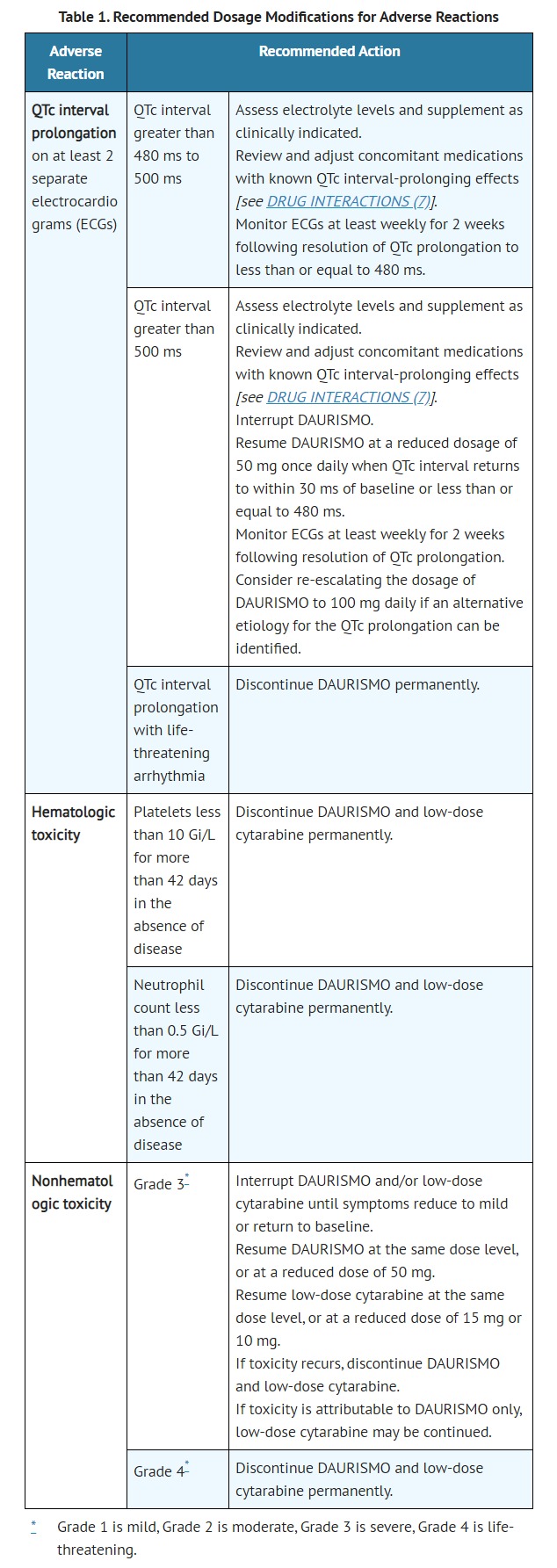
- ADVERSE REACTIONS
- Clinical Trials Experience
- Dose reductions associated with adverse reactions were reported in 26% of patients treated with DAURISMO with low-dose cytarabine, and the most common reasons (≥2%) for dose reductions due to adverse reactions were muscle spasms (5%), fatigue (4%), febrile neutropenia (4%), anemia (2%), thrombocytopenia (2%), and ECG QT prolonged (2%). Adverse reactions leading to permanent discontinuation were reported in 36% of patients treated with DAURISMO with low-dose cytarabine, and the most common (≥2%) reasons for permanent discontinuation were pneumonia (6%), febrile neutropenia (4%), sepsis (4%), sudden death (2%), myocardial infarction (2%), nausea (2%), and renal insufficiency (2%).
- Additional clinically-significant adverse reactions occurring in < 10% of patients treated with DAURISMO and low-dose cytarabine in BRIGHT AML 1003 include:
- Dental disorders: loose tooth and toothache
- Skin and subcutaneous tissue disorders: alopecia
- Cardiac disorders: QT interval prolonged
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Cardiac Electrophysiology
- The effect of glasdegib administration on corrected QT interval (QTc) was evaluated in a randomized, single-dose, double-blind, 4-way crossover, placebo- and open-label moxifloxacin-controlled study in 36 healthy subjects. At therapeutic plasma concentrations for the recommended dose, achieved with a single dose of 150 mg DAURISMO, the largest placebo and baseline-adjusted QTc interval change was 8 ms (90% CI: 6, 10 ms). At a two-fold therapeutic plasma concentration, achieved with a single dose of 300 mg DAURISMO, the QTc change was 13 ms (90% CI: 11, 16 ms). Glasdegib is associated with concentration-dependent QTc prolongation.
- PATIENT COUNSELING INFORMATION
- QT Interval Prolongation
- Inform patients of signs and symptoms that may be indicative of significant QT interval prolongation. Advise patients to contact their healthcare provider immediately in the event of syncope, pre-syncopal symptoms, and cardiac palpitations [see WARNINGS AND PRECAUTIONS (5.2)].
- MEDICATION GUIDE
- What are the possible side effects of DAURISMO?
- DAURISMO can cause serious side effects, including:
- See "WHAT IS THE MOST IMPORTANT INFORMATION I SHOULD KNOW ABOUT DAURISMO?"
- Changes in the electrical activity of your heart called QT prolongation. QT prolongation can cause irregular heartbeats that can be life-threatening. Tell your healthcare provider right away if you feel faint, lightheaded, dizzy, or feel your heart beating irregularly or fast during treatment with DAURISMO.
- USE IN SPECIFIC POPULATIONS
- Renal Impairment
- No dosage modification is recommended for patients with mild to severe renal impairment (estimated glomerular filtration rate [eGFR] 15 to 89 mL/min). Monitor patients with severe renal impairment (eGFR 15 to 29 mL/min) for increased risk of adverse reactions, including QTc interval prolongation, due to increased glasdegib concentrations [see CLINICAL PHARMACOLOGY (12.3)].
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
5
24087
Other ADRs
349
38381238
Odds Ratio = 22.829
Drug Property Information
ATC Code(s):
- L01XJ03 - glasdegib
- L01XJ0 -
- L01XJ -
- L01X - OTHER ANTINEOPLASTIC AGENTS
- L01 - ANTINEOPLASTIC AGENTS
- L - ANTINEOPLASTIC AND IMMUNOMODULATING AGENTS
Active Ingredient:GLASDEGIB
Active Ingredient UNII:K673DMO5H9
Drugbank ID:DB11978
PubChem Compound:25166913
CTD ID:C000592580
CAS Number:1095173-27-5
Dosage Form(s):tablet, film coated
Route(s) Of Administrator:oral
Daily Dose:
Chemical Structure: 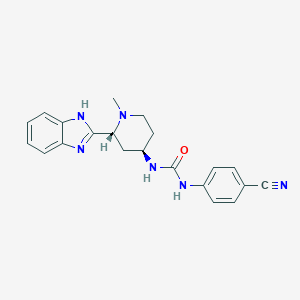

SMILE Code:
CN1CC[C@H](C[C@@H]1C1=NC2=CC=CC=C2N1)NC(=O)NC1=CC=C(C=C1)C#N
CN1CC[C@H](C[C@@H]1C1=NC2=CC=CC=C2N1)NC(=O)NC1=CC=C(C=C1)C#N
Reference
1: Drug-drug interactions of newly approved small molecule inhibitors for acute myeloid leukemia.
[Megías-Vericat Juan Eduardo,Solana-Altabella Antonio,Ballesta-López Octavio,Martínez-Cuadrón David,Montesinos Pau]Ann Hematol,2020 Sep;99(9):1989-2007. PMID: 32683457
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.