Search for drugs:
Typing the drug name to query
CARBINOXAMINE MALEATE
DIR Classification
Classification:Ambiguous
Severity Score:1.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- CLINICAL PHARMACOLOGY
- Cardiac Electrophysiology
- Cardiac effects, including prolongation of QT interval have not been adequately studied. Unlike other newer antihistamines, severe adverse cardiovascular effects are uncommon, and usually limited to over dosage situations.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
0
24092
Other ADRs
85
38381502
Odds Ratio = 0.0
Drug Property Information
ATC Code(s):
- R06AA08 - carbinoxamine maleate
- R06AA - Aminoalkyl ethers
- R06A - ANTIHISTAMINES FOR SYSTEMIC USE
- R06 - ANTIHISTAMINES FOR SYSTEMIC USE
- R - RESPIRATORY SYSTEM
Active Ingredient:CARBINOXAMINE MALEATE
Active Ingredient UNII:02O55696WH
Drugbank ID:DB00748
PubChem Compound:2564
CTD ID:C004649
PharmGKB:PA164746898
CAS Number:486-16-8
Dosage Form(s):syrup; tablet
Route(s) Of Administrator:oral
Daily Dose:
- 16.0 mg/day R06AA08
Chemical Structure: 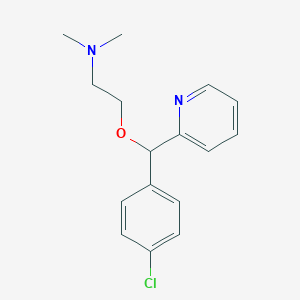

SMILE Code:
CN(C)CCOC(C1=CC=C(Cl)C=C1)C1=CC=CC=N1
CN(C)CCOC(C1=CC=C(Cl)C=C1)C1=CC=CC=N1
Reference
N/A
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.