Search for drugs:
Typing the drug name to query
PIMAVANSERIN TARTRATE
DIR Classification
Classification:Most-DIQT concern
Severity Score:4.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
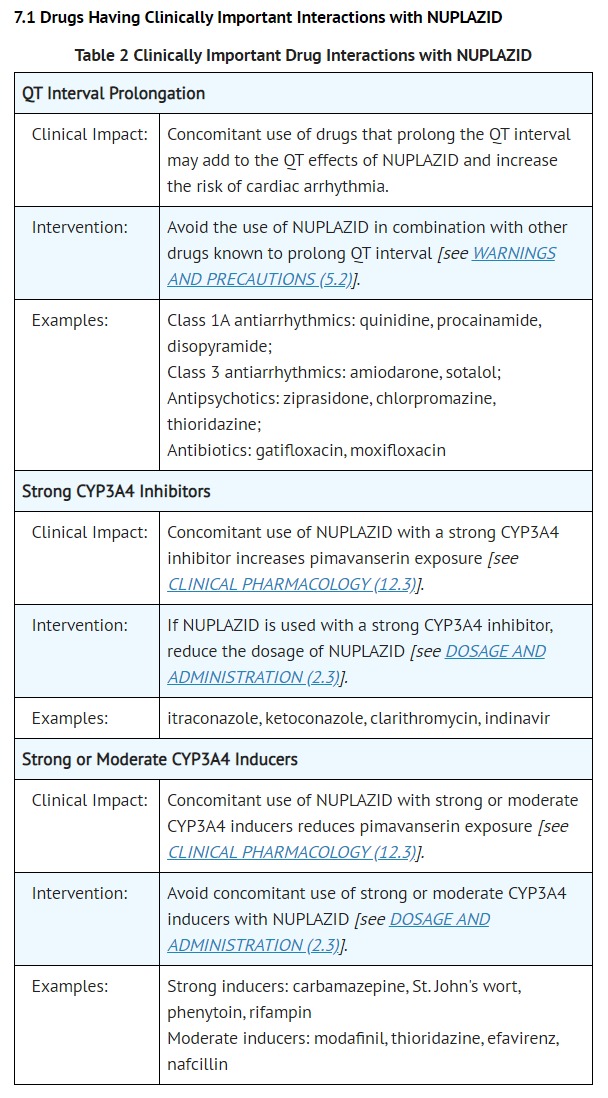
- WARNINGS AND PRECAUTIONS
- QT Interval Prolongation
- NUPLAZID prolongs the QT interval. The use of NUPLAZID should be avoided in patients with known QT prolongation or in combination with other drugs known to prolong QT interval including Class 1A antiarrhythmics (e.g., quinidine, procainamide) or Class 3 antiarrhythmics (e.g., amiodarone, sotalol), certain antipsychotic medications (e.g., ziprasidone, chlorpromazine, thioridazine), and certain antibiotics (e.g., gatifloxacin, moxifloxacin) [see DRUG INTERACTIONS (7.1)]. NUPLAZID should also be avoided in patients with a history of cardiac arrhythmias, as well as other circumstances that may increase the risk of the occurrence of torsade de pointes and/or sudden death, including symptomatic bradycardia, hypokalemia or hypomagnesemia, and the presence of congenital prolongation of the QT interval [see CLINICAL PHARMACOLOGY (12.2)].
- DRUG INTERACTIONS
- OVERDOSAGE
- Management of Overdose
- There are no known specific antidotes for NUPLAZID. In managing overdose, cardiovascular monitoring should commence immediately and should include continuous ECG monitoring to detect possible arrhythmias [see WARNINGS AND PRECAUTIONS (5.2)]. If antiarrhythmic therapy is administered, disopyramide, procainamide, and quinidine should not be used, as they have the potential for QT-prolonging effects that might be additive to those of NUPLAZID [see DRUG INTERACTIONS (7.1)]. Consider the long plasma half-life of pimavanserin (about 57 hours) and the possibility of multiple drug involvement. Consult a Certified Poison Control Center (1-800-222-1222) for up-to-date guidance and advice.
- ADVERSE REACTIONS
- The following serious adverse reactions are discussed elsewhere in the labeling:
- Increased Mortality in Elderly Patients with Dementia-Related Psychosis [see BOXED WARNING and WARNINGS AND PRECAUTIONS (5.1)]
- QT Interval Prolongation [see WARNINGS AND PRECAUTIONS (5.2)]
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Cardiac Electrophysiology
- The effect of NUPLAZID on the QTc interval was evaluated in a randomized placebo- and positive-controlled double-blind, multiple-dose parallel thorough QTc study in 252 healthy subjects. A central tendency analysis of the QTc data at steady-state demonstrated that the maximum mean change from baseline (upper bound of the two-sided 90% CI) was 13.5 (16.6) msec at a dose of twice the therapeutic dose. A pharmacokinetic/ pharmacodynamic analysis with NUPLAZID suggested a concentration-dependent QTc interval prolongation in the therapeutic range.
- In the 6-week, placebo-controlled effectiveness studies, mean increases in QTc interval of ~5-8 msec were observed in patients receiving once-daily doses of NUPLAZID 34 mg. These data are consistent with the profile observed in a thorough QT study in healthy subjects. Sporadic QTcF values ≥500 msec and change from baseline values ≥60 msec were observed in subjects treated with NUPLAZID 34 mg; although the incidence was generally similar for NUPLAZID and placebo groups. There were no reports of torsade de pointes or any differences from placebo in the incidence of other adverse reactions associated with delayed ventricular repolarization in studies of NUPLAZID, including those patients with hallucinations and delusions associated with PDP [see WARNINGS AND PRECAUTIONS (5.2)].
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
35
24057
Other ADRs
50744
38330843
Odds Ratio = 1.099
Drug Property Information
ATC Code(s):
- N05AX17 - pimavanserin tartrate
- N05AX - Other antipsychotics
- N05A - ANTIPSYCHOTICS
- N05 - PSYCHOLEPTICS
- N - NERVOUS SYSTEM
Active Ingredient:pimavanserin tartrate
Active Ingredient UNII:NA83F1SJSR
Drugbank ID:DB05316
PubChem Compound:10071196
CTD ID:C510793
CAS Number:706779-91-1
Dosage Form(s):capsule; tablet, coated
Route(s) Of Administrator:oral
Daily Dose:
Chemical Structure: 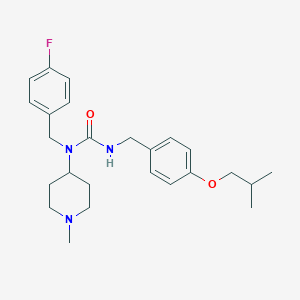

SMILE Code:
CC(C)COC1=CC=C(CNC(=O)N(CC2=CC=C(F)C=C2)C2CCN(C)CC2)C=C1
CC(C)COC1=CC=C(CNC(=O)N(CC2=CC=C(F)C=C2)C2CCN(C)CC2)C=C1
Reference
N/A
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.