Search for drugs:
Typing the drug name to query
MACITENTAN
DIR Classification
Classification:Ambiguous
Severity Score:1.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Cardiac Electrophysiology: In a randomized, placebo-controlled four-way crossover study with a positive control in healthy subjects, repeated doses of macitentan 10 and 30 mg (3 times the recommended dosage) had no significant effect on the QTc interval.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
9
24083
Other ADRs
134980
38246607
Odds Ratio = 0.106
Drug Property Information
ATC Code(s):
- C02KX04 - macitentan
- C02KX - Other antihypertensives
- C02K - OTHER ANTIHYPERTENSIVES
- C02 - ANTIHYPERTENSIVES
- C - CARDIOVASCULAR SYSTEM
Active Ingredient:macitentan
Active Ingredient UNII:Z9K9Y9WMVL
Drugbank ID:DB08932
PubChem Compound:16004692
CTD ID: C533860
CAS Number:441798-33-0
Dosage Form(s):tablet, film coated
Route(s) Of Administrator:oral
Daily Dose:
- 10.0 mg/day C02KX04
Chemical Structure: 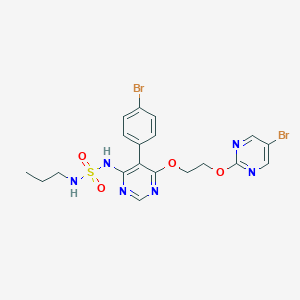

SMILE Code:
CCCNS(=O)(=O)NC1=C(C(OCCOC2=NC=C(Br)C=N2)=NC=N1)C1=CC=C(Br)C=C1
CCCNS(=O)(=O)NC1=C(C(OCCOC2=NC=C(Br)C=N2)=NC=N1)C1=CC=C(Br)C=C1
Reference
1: Macitentan, a dual endothelin receptor antagonist for the treatment of pulmonary arterial hypertension, does not affect cardiac repolarization in healthy subjects.
[Lindegger Nicolas,Sidharta Patricia N,Reseski Kathrin,Dingemanse Jasper]Pulm Pharmacol Ther,2014 Oct;29(1):41-8. PMID: 24813561
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.