Search for drugs:
Typing the drug name to query
DOXEPIN HYDROCHLORIDE
DIR Classification
Classification:Ambiguous
Severity Score:1.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Cardiac electrophysiology
- In a thorough QTc prolongation clinical study in healthy subjects, doxepin had no effect on QT intervals or other electrocardiographic parameters after multiple daily doses up to 50 mg.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
14
24078
Other ADRs
4592
38376995
Odds Ratio = 4.86
Drug Property Information
ATC Code(s):
- N06AA12 - doxepin hydrochloride
- N06AA - Non-selective monoamine reuptake inhibitors
- N06A - ANTIDEPRESSANTS
- N06 - PSYCHOANALEPTICS
- N - NERVOUS SYSTEM
- D04AX01 - doxepin hydrochloride
- D04AX - Other antipruritics
- D04A - "ANTIPRURITICS, INCL. ANTIHISTAMINES, ANESTHETICS, ETC."
- D04 - "ANTIPRURITICS, INCL. ANTIHISTAMINES, ANESTHETICS, ETC."
- D - DERMATOLOGICALS
Active Ingredient:doxepin hydrochloride
Active Ingredient UNII:3U9A0FE9N5
Drugbank ID:DB01142
PubChem Compound:667468
CTD ID:D004316
PharmGKB:PA449409
CAS Number:1668-19-5
Dosage Form(s):tablet
Route(s) Of Administrator:oral
Daily Dose:
- 100.0 mg/day N06AA12
Chemical Structure: 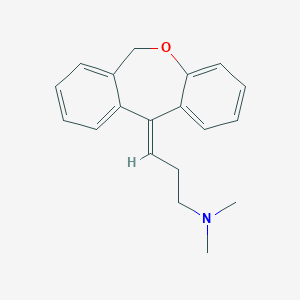

SMILE Code:
[H]C(CCN(C)C)=C1C2=CC=CC=C2COC2=CC=CC=C12
[H]C(CCN(C)C)=C1C2=CC=CC=C2COC2=CC=CC=C12
Reference
N/A
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.