Search for drugs:
Typing the drug name to query
LETERMOVIR
DIR Classification
Classification:Most-DIQT concern
Severity Score:4.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- DRUG INTERACTIONS
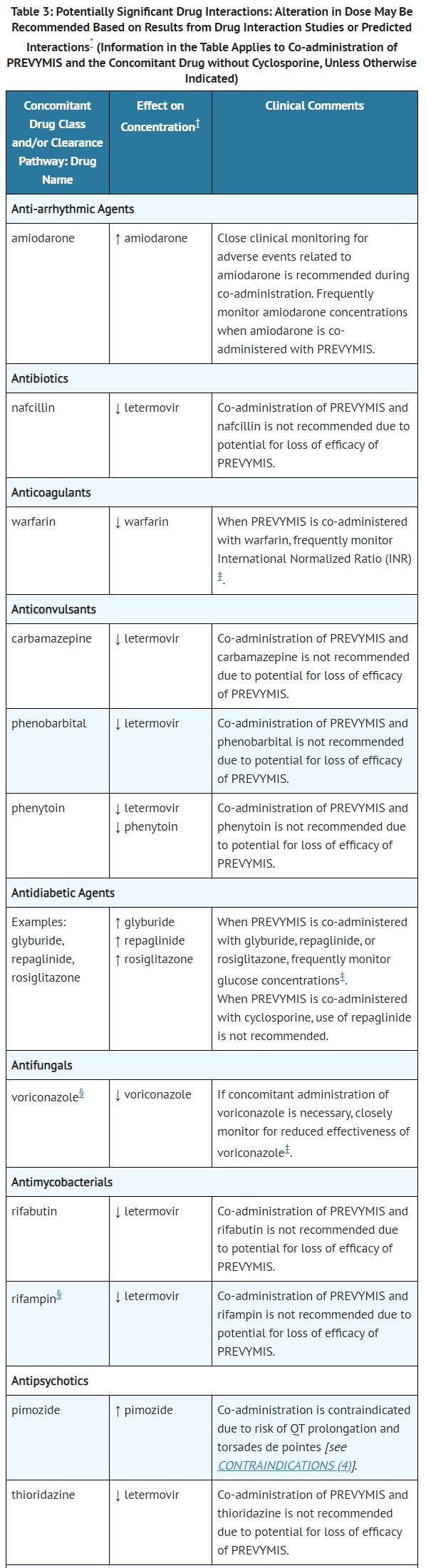
- CONTRAINDICATIONS
- PREVYMIS is contraindicated in patients receiving pimozide or ergot alkaloids:
- Pimozide: Concomitant administration of PREVYMIS in patients receiving pimozide may result in increased concentrations of pimozide due to inhibition of cytochrome P450 3A (CYP3A) by letermovir, which may lead to QT prolongation and torsades de pointes [see WARNINGS AND PRECAUTIONS (5.1) and DRUG INTERACTIONS (7.2, 7.3)].
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Cardiac Electrophysiology
- In a thorough QT trial in healthy subjects, letermovir at the therapeutic IV dose or at a dose of 2 times the approved IV dose did not prolong QTc to any clinically relevant extent.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
0
24092
Other ADRs
788
38380799
Odds Ratio = 0.0
Drug Property Information
ATC Code(s):
- J05AX18 - letermovir
- J05AX - Other antivirals
- J05A - DIRECT ACTING ANTIVIRALS
- J05 - ANTIVIRALS FOR SYSTEMIC USE
- J - ANTIINFECTIVES FOR SYSTEMIC USE
Active Ingredient:LETERMOVIR
Active Ingredient UNII:1H09Y5WO1F
Drugbank ID:DB12070
PubChem Compound:N/ADIR Classification
CTD ID:C000588473
CAS Number:917389-32-3
Dosage Form(s):injection, solution; tablet, film coated
Route(s) Of Administrator:intravenous; oral
Daily Dose:
- 480.0 mg/day J05AX18
Chemical Structure: 

SMILE Code:
COC1=CC=CC(=C1)N1CCN(CC1)C1=NC2=C(C=CC=C2F)[C@H](CC(O)=O)N1C1=CC(=CC=C1OC)C(F)(F)F
COC1=CC=CC(=C1)N1CCN(CC1)C1=NC2=C(C=CC=C2F)[C@H](CC(O)=O)N1C1=CC(=CC=C1OC)C(F)(F)F
Reference
N/A
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.