Search for drugs:
Typing the drug name to query
LOMEFLOXACIN HYDROCHLORIDE
DIR Classification
Classification:Most-DIQT concern
Severity Score:4.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- WARNINGS
- QT interval prolongation/torsades de pointes
- Rare cases of torsades de pointes have been spontaneously reported during post-marketing surveillance in patients receiving quinolones, including lomefloxacin. These rare cases were associated with one or more of the following factors: age over 60, female gender, underlying cardiac disease, and/or use of multiple medications. Lomefloxacin should be avoided in patients with known prolongation of the QT interval, patients with uncorrected hypokalemia, and patients receiving class IA (quinidine, procainamide), or class III (amiodarone, sotalol) antiarrhythmic agents.
- ADVERSE REACTIONS
- Quinolone-class adverse events
- Additional quinolone-class adverse events include: peripheral neuropathy, torsades de pointes, erythema nodosum, hepatic necrosis, possible exacerbation of myasthenia gravis, dysphasia, nystagmus, intestinal perforation, manic reaction, renal calculi, acidosis and hiccough.
- Laboratory adverse events include: agranulocytosis, elevation of serum triglycerides, elevation of serum cholesterol, elevation of blood glucose, elevation of serum potassium, albuminuria, candiduria, and crystalluria.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
0
24092
Other ADRs
86
38381501
Odds Ratio = 0.0
Drug Property Information
ATC Code(s):
- J01MA07 - lomefloxacin hydrochloride
- J01MA - Fluoroquinolones
- J01M - QUINOLONE ANTIBACTERIALS
- J01 - ANTIBACTERIALS FOR SYSTEMIC USE
- J - ANTIINFECTIVES FOR SYSTEMIC USE
- S01AE04 - lomefloxacin hydrochloride
- S01AE -
- S01A - ANTIINFECTIVES
- S01 - OPHTHALMOLOGICALS
- S - SENSORY ORGANS
Active Ingredient:lomefloxacin hydrochloride
Active Ingredient UNII:9VC7S3ZXXB
Drugbank ID:DB00978
PubChem Compound:3948
CTD ID:C053091
PharmGKB:PA164749165
CAS Number:98079-51-7
Dosage Form(s):tablet, film coated
Route(s) Of Administrator:oral
Daily Dose:
- 400.0 mg/day J01MA07
Chemical Structure: 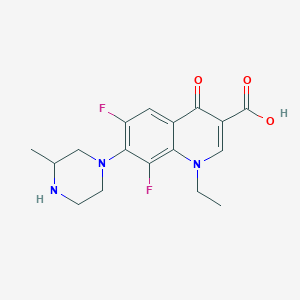

SMILE Code:
CCN1C=C(C(O)=O)C(=O)C2=CC(F)=C(N3CCNC(C)C3)C(F)=C12
CCN1C=C(C(O)=O)C(=O)C2=CC(F)=C(N3CCNC(C)C3)C(F)=C12
Reference
N/A
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.