Search for drugs:
Typing the drug name to query
PONATINIB HYDROCHLORIDE
DIR Classification
Classification:Moderate-DIQT concern
Severity Score:2.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- WARNINGS AND PRECAUTIONS
- Cardiac Arrhythmias
- Atrial fibrillation was the most common arrhythmia and occurred in 7% (31/449) of patients, approximately half of which were Grade 3 or 4. Other Grade 3 or 4 arrhythmia events included syncope (9 patients; 2.0%), tachycardia and bradycardia (2 patients each 0.4%), and electrocardiogram QT prolonged, atrial flutter, supraventricular tachycardia, ventricular tachycardia, atrial tachycardia, atrioventricular block complete, cardio-respiratory arrest, loss of consciousness, and sinus node dysfunction (1 patient each 0.2%). For 27 patients, the event led to hospitalization.
- OVERDOSAGE
- Overdoses with Iclusig were reported in clinical trials. One patient was accidentally administered the entire contents of a bottle of study medication via nasogastric tube. The investigator estimated that the patient received 540 mg of Iclusig. Two hours after the overdose, the patient had an uncorrected QT interval of 520 ms. Subsequent ECGs showed normal sinus rhythm with uncorrected QT intervals of 480 ms and 400 ms. The patient died 9 days after the overdose from pneumonia and sepsis. Another patient accidentally self-administered 165 mg on Cycle 1 Day 2. The patient experienced fatigue and non-cardiac chest pain on Day 3. Multiple doses of 90 mg per day for 12 days in a patient resulted in pneumonia, systemic inflammatory response, atrial fibrillation, and a moderate pericardial effusion.
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Cardiac Electrophysiology
- A QT assessment was performed in 39 patients with cancer who received 30 mg, 45 mg, or 60 mg Iclusig once daily. No large changes in the mean QTc interval (i.e., >20 msec) from baseline were detected in the study. However, a small increase in the mean QTc interval (i.e., <10 msec) cannot be excluded because of study design limitations. In a Phase 3 trial comparing ponatinib with imatinib, the mean change from baseline to worst QTcF value in ponatinib-treated patients (n=124) was <10 msec.
- MEDICATION GUIDE
- Before you take Iclusig, tell your healthcare provider about all of your medical conditions, including if you:
- have a history of blood clots in your blood vessels (arteries or veins)
- have heart problems, including heart failure, irregular heartbeats, and QT prolongation
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
11
24081
Other ADRs
26645
38354942
Odds Ratio = 0.658
Drug Property Information
ATC Code(s):
- L01EA05 - ponatinib hydrochloride
- L01EA -
- L01E -
- L01 - ANTINEOPLASTIC AGENTS
- L - ANTINEOPLASTIC AND IMMUNOMODULATING AGENTS
Active Ingredient:ponatinib hydrochloride
Active Ingredient UNII:96R6PU3D8J
Drugbank ID:DB08901
PubChem Compound:24826799
CTD ID:C545373
PharmGKB:PA165980594
CAS Number:943319-70-8
Dosage Form(s):tablet, film coated
Route(s) Of Administrator:oral
Daily Dose:
Chemical Structure: 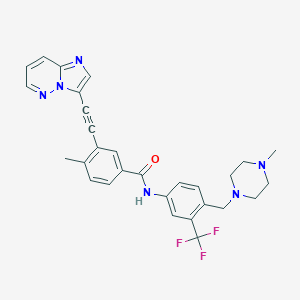

SMILE Code:
CN1CCN(CC2=CC=C(NC(=O)C3=CC(C#CC4=CN=C5C=CC=NN45)=C(C)C=C3)C=C2C(F)(F)F)CC1
CN1CCN(CC2=CC=C(NC(=O)C3=CC(C#CC4=CN=C5C=CC=NN45)=C(C)C=C3)C=C2C(F)(F)F)CC1
Reference
1: Cardiovascular Toxicity of Tyrosine Kinase Inhibitors Used in Chronic Myeloid Leukemia: An Analysis of the FDA Adverse Event Reporting System Database (FAERS).
[Cirmi Santa,El Abd Asmae,Letinier Louis,Navarra Michele,Salvo Francesco]Cancers (Basel),2020 Mar 30;12(4):826. PMID: 32235443
2: Cardiovascular Complications of Targeted Therapies for Chronic Myeloid Leukemia.
[Damrongwatanasuk Rongras,Fradley Michael G]Curr Treat Options Cardiovasc Med,2017 Apr;19(4):24. PMID: 28316033
3: Update on Cardiovascular Safety of Tyrosine Kinase Inhibitors: With a Special Focus on QT Interval, Left Ventricular Dysfunction and Overall Risk/Benefit.
[Shah Rashmi R,Morganroth Joel]Drug Saf,2015 Aug;38(8):693-710. PMID: 26008987
4: QTc interval prolongation with vascular endothelial growth factor receptor tyrosine kinase inhibitors.
[Ghatalia P,Je Y,Kaymakcalan M D,Sonpavde G,Choueiri T K]Br J Cancer,2015 Jan 20;112(2):296-305. PMID: 25349964
5: Analysis of the potential effect of ponatinib on the QTc interval in patients with refractory hematological malignancies.
[Sonnichsen Daryl,Dorer David J,Cortes Jorge,Talpaz Moshe,Deininger Michael W,Shah Neil P,Kantarjian Hagop M,Bixby Dale,Mauro Michael J,Flinn Ian W,Litwin Jeffrey,Turner Christopher D,Haluska Frank G]Cancer Chemother Pharmacol,2013 Jun;71(6):1599-607. PMID: 23609479
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.