Search for drugs:
Typing the drug name to query
VORICONAZOLE
DIR Classification
Classification:Most-DIQT concern
Severity Score:4.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- WARNINGS AND PRECAUTIONS
- Arrhythmias and QT Prolongation
- Some azoles, including voriconazole, have been associated with prolongation of the QT interval on the electrocardiogram. During clinical development and post-marketing surveillance, there have been rare cases of arrhythmias, (including ventricular arrhythmias such as torsade de pointes), cardiac arrests and sudden deaths in patients taking voriconazole. These cases usually involved seriously ill patients with multiple confounding risk factors, such as history of cardiotoxic chemotherapy, cardiomyopathy, hypokalemia and concomitant medications that may have been contributory.
- Voriconazole should be administered with caution to patients with potentially proarrhythmic conditions, such as:
- Congenital or acquired QT prolongation
- Cardiomyopathy, in particular when heart failure is present
- Sinus bradycardia
- Existing symptomatic arrhythmias
- Concomitant medicinal product that is known to prolong QT interval [see contraindications (4), Drug Interactions (7), and Clinical Pharmacology (12.3)]
- Rigorous attempts to correct potassium, magnesium and calcium should be made before starting and during voriconazole therapy [see Clinical Pharmacology (12.3)].
- DRUG INTERACTIONS
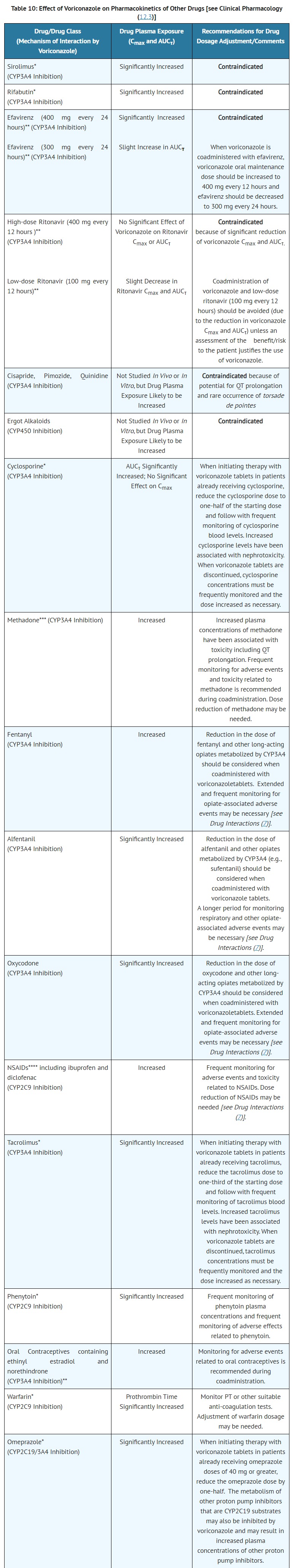
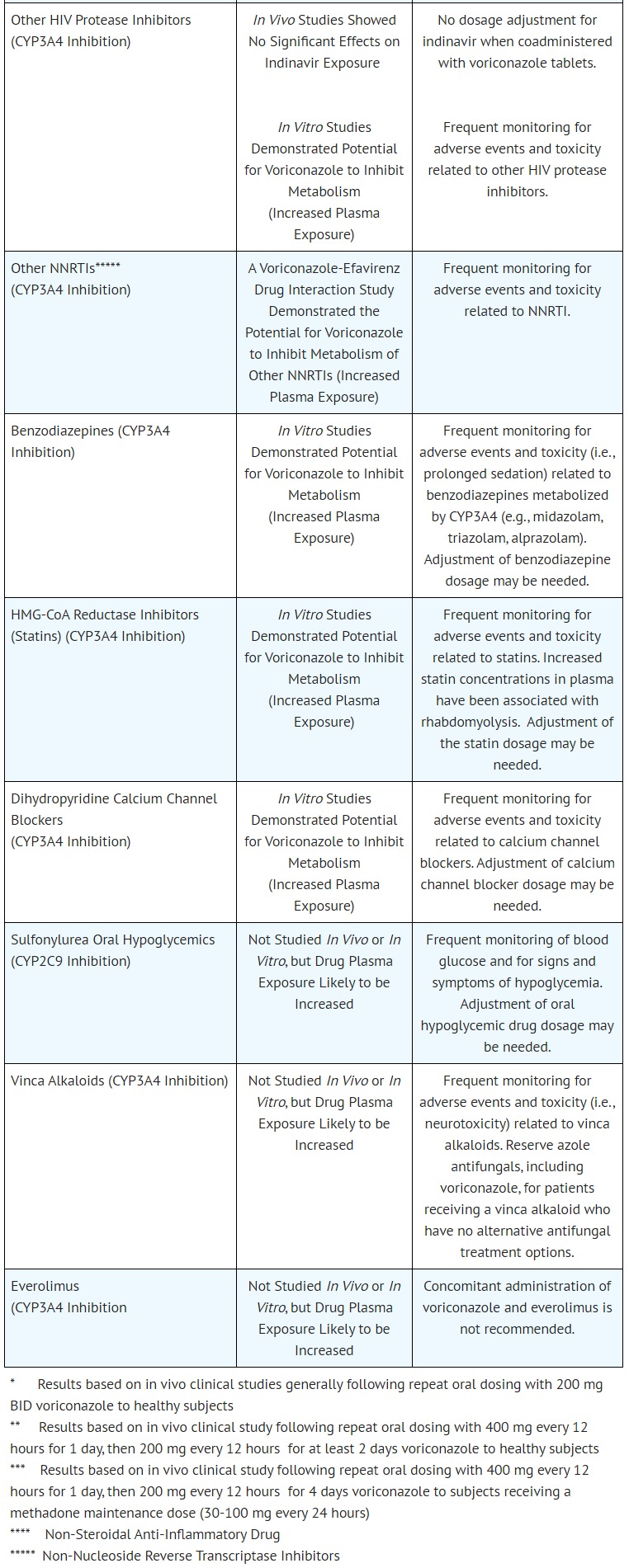
- CONTRAINDICATIONS
- Coadministration of cisapride, pimozide or quinidine with voriconazole tablets is contraindicated because increased plasma concentrations of these drugs can lead to QT prolongation and rare occurrences of torsade de pointes [see Drug Interactions (7) and Clinical Pharmacology (12.3)].
- ADVERSE REACTIONS
- Cardiovascular: atrial arrhythmia, atrial fibrillation, AV block complete, bigeminy, bradycardia, bundle branch block, cardiomegaly, cardiomyopathy, cerebral hemorrhage, cerebral ischemia, cerebrovascular accident, congestive heart failure, deep thrombophlebitis, endocarditis, extrasystoles, heart arrest, hypertension, hypotension, myocardial infarction, nodal arrhythmia, palpitation, phlebitis, postural hypotension, pulmonary embolus, QT interval prolonged, supraventricular extrasystoles, supraventricular tachycardia, syncope, thrombophlebitis, vasodilatation, ventricular arrhythmia, ventricular fibrillation, ventricular tachycardia (including torsade de pointes) [see Warnings and Precautions (5.2)].
- CLINICAL PHARMACOLOGY
- Cardiac Electrophysiology
- A placebo-controlled, randomized, crossover study to evaluate the effect on the QT interval of healthy male and female subjects was conducted with three single oral doses of voriconazole and ketoconazole. Serial ECGs and plasma samples were obtained at specified intervals over a 24-hour post dose observation period. The placebo-adjusted mean maximum increases in QTc from baseline after 800, 1200, and 1600 mg of voriconazole and after ketoconazole 800 mg were all <10 msec. Females exhibited a greater increase in QTc than males, although all mean changes were <10 msec. Age was not found to affect the magnitude of increase in QTc. No subject in any group had an increase in QTc of ≥60 msec from baseline. No subject experienced an interval exceeding the potentially clinically relevant threshold of 500 msec. However, the QT effect of voriconazole combined with drugs known to prolong the QT interval is unknown [see Contraindications (4) and Drug Interactions (7)].
- [Pharmacokinetics]
- Cisapride, pimozide and quinidine (CYP3A4 substrates)–Although not studied in vitro or in vivo, concomitant administration of voriconazole with cisapride, pimozide or quinidine may result in inhibition of the metabolism of these drugs. Increased plasma concentrations of these drugs can lead to QT prolongation and rare occurrences of torsade de pointes. Coadministration of voriconazole, cisapride, pimozide and quinidine is contraindicated [see Contraindications (4) and Warnings and Precautions (5.12)].
- Methadone (CYP3A4, CYP2C19, CYP2C9 substrate)–Repeat dose administration of oral voriconazole (400 mg every 12 hours for 1 day, then 200 mg every 12 hours for 4 days) increased the Cmax and AUCτ of pharmacologically active Rmethadone by 31% (90% CI: 22%, 40%) and 47% (90% CI: 38%, 57%), respectively, in subjects receiving a methadone maintenance dose (30-100 mg every 24 hours). The Cmax and AUC of (S)-methadone increased by 65% (90% CI: 53%, 79%) and 103% (90% CI: 85%, 124%), respectively. Increased plasma concentrations of methadone have been associated with toxicity including QT prolongation. Frequent monitoring for adverse events and toxicity related to methadone is recommended during coadministration. Dose reduction of methadone may be needed [see Warnings and Precautions (5.12)].
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
66
24026
Other ADRs
26066
38355521
Odds Ratio = 4.043
Drug Property Information
ATC Code(s):
- J02AC03 - voriconazole
- J02AC - Triazole derivatives
- J02A - ANTIMYCOTICS FOR SYSTEMIC USE
- J02 - ANTIMYCOTICS FOR SYSTEMIC USE
- J - ANTIINFECTIVES FOR SYSTEMIC USE
Active Ingredient:Voriconazole; voriconazole
Active Ingredient UNII:JFU09I87TR
Drugbank ID:DB00582
PubChem Compound:71616
CTD ID:D065819
PharmGKB:PA10233
CAS Number:137234-62-9
Dosage Form(s):tablet
Route(s) Of Administrator:oral
Daily Dose:
- 400.0 mg/day J02AC03
Chemical Structure: 

SMILE Code:
C[C@@H](C1=NC=NC=C1F)[C@](O)(CN1C=NC=N1)C1=C(F)C=C(F)C=C1
C[C@@H](C1=NC=NC=C1F)[C@](O)(CN1C=NC=N1)C1=C(F)C=C(F)C=C1
Reference
1: Characterization of Therapeutic Drug Monitoring Practices of Voriconazole and Posaconazole at a Pediatric Hospital.
[Duehlmeyer Stephanie,Klockau Christopher,Yu Diana,Rouch Jamie]J Pediatr Pharmacol Ther,2021;26(1):26-32. PMID: 33424497
2: QT prolongation in patients with acute leukemia or high-risk myelodysplastic syndrome prescribed antifungal prophylaxis during chemotherapy-induced neutropenia.
[Barreto Jason N,Cullen Michael W,Mara Kristin C,Grove Meagan E,Sierzchulski Amanda G,Dahl Nathan J,Tosh Pritish K,Dierkhising Ross A,Patnaik Mrinal M,Ackerman Michael J]Leuk Lymphoma,2019 Dec;60(14):3512-3520. PMID: 31298598
3: Comparative evaluation of isavuconazonium sulfate, voriconazole, and posaconazole for the management of invasive fungal infections in an academic medical center.
[Van Matre Edward T,Evans Shelby L,Mueller Scott W,MacLaren Robert,Fish Douglas N,Kiser Tyree H]Ann Clin Microbiol Antimicrob,2019 Mar 20;18(1):13. PMID: 30894179
4: Voriconazole-induced QTc prolongation in a paediatric population.
[Pasternak Y,Shechter N,Loebstein R,Markovits N,Gueta I,Halkin H,Yarden-Bilavsky H]Acta Paediatr,2019 Jun;108(6):1128-1132. PMID: 30456871
5: Real-world implications of QT prolongation in patients receiving voriconazole and amiodarone.
[Mourad Ahmad,Stiber Jonathan A,Perfect John R,Johnson Melissa D]J Antimicrob Chemother,2019 Jan 1;74(1):228-233. PMID: 30295798
6: Treatment of Chronic Pulmonary Aspergillosis: Current Standards and Future Perspectives.
[Alastruey-Izquierdo Ana,Cadranel Jacques,Flick Holger,Godet Cendrine,Hennequin Christophe,Hoenigl Martin,Kosmidis Chris,Lange Christoph,Munteanu Oxana,Page Iain,Salzer Helmut J F,on behalf of CPAnet]Respiration,2018;96(2):159-170. PMID: 29982245
7: Issues in the management of invasive pulmonary aspergillosis in non-neutropenic patients in the intensive care unit: A role for isavuconazole.
[Bassetti Matteo,Carnelutti Alessia,Righi Elda]IDCases,2018 Mar 1;12:7-9. PMID: 29850401
8: Torsade de pointes and systemic azole antifungal agents: Analysis of global spontaneous safety reports.
[Salem M,Reichlin T,Fasel D,Leuppi-Taegtmeyer A]Glob Cardiol Sci Pract,2017 Jun 30;2017(2):11. PMID: 29644223
9: Voriconazole-induced QT prolongation among hemato-oncologic patients: clinical characteristics and risk factors.
[Gueta I,Loebstein R,Markovits N,Kamari Y,Halkin H,Livni G,Yarden-Bilavsky H]Eur J Clin Pharmacol,2017 Sep;73(9):1181-1185. PMID: 28624887
10: Use of isavuconazole in a patient with voriconazole-induced QTc prolongation.
[Trang Tracy P,Hanretty Alexandra M,Langelier Charles,Yang Katherine]Transpl Infect Dis,2017 Aug;19(4). PMID: 28434195
11: Role of isavuconazole in the treatment of invasive fungal infections.
[Wilson Dustin T,Dimondi V Paul,Johnson Steven W,Jones Travis M,Drew Richard H]Ther Clin Risk Manag,2016 Aug 3;12:1197-206. PMID: 27536124
12: Divergent electrophysiologic profile of fluconazole and voriconazole in an experimental whole-heart model of proarrhythmia.
[Frommeyer Gerrit,Fischer Christina,Lange Philipp S,Leitz Patrick,Fehr Michael,Bogossian Harilaos,Milberg Peter,Eckardt Lars]Eur J Pharmacol,2016 Apr 5;776:185-90. PMID: 26905475
13: Voriconazole associated torsades de pointes in two adult patients with haematological malignancies.
[Brown Jeremy D,Lim Lyn-Li,Koning Sonia]Med Mycol Case Rep,2014 Mar 13;4:23-5. PMID: 24855597
14: Pharmacokinetics of intravenous voriconazole in obese patients: implications of CYP2C19 homozygous poor metabolizer genotype.
[Moriyama Brad,Jarosinski Paul F,Figg William D,Henning Stacey A,Danner Robert L,Penzak Scott R,Wayne Alan S,Walsh Thomas J]Pharmacotherapy,2013 Mar;33(3):e19-22. PMID: 23400848
15: Effect of combined fluoroquinolone and azole use on QT prolongation in hematology patients.
[Zeuli John D,Wilson John W,Estes Lynn L]Antimicrob Agents Chemother,2013 Mar;57(3):1121-7. PMID: 23229485
16: OTc prolongation and torsade de pointes ventricular tachycardia in a small dose voriconazole therapy.
[Elbey M A,Cil H,Onturk E,Islamoglu Y]Eur Rev Med Pharmacol Sci,2012 Jan;16(1):100-2. PMID: 22338554
17: Torsade de pointes caused by polypharmacy and substance abuse in a patient with human immunodeficiency virus.
[Prosser Jane M,Mills Angela,Rhim Eugene S,Perrone Jeanmarie]Int J Emerg Med,2008 Sep;1(3):217-20. PMID: 19384521
18: Torsades de pointes associated with voriconazole use.
[Philips J A,Marty F M,Stone R M,Koplan B A,Katz J T,Baden L R]Transpl Infect Dis,2007 Mar;9(1):33-6. PMID: 17313469
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.