Search for drugs:
Typing the drug name to query
BORTEZOMIB
DIR Classification
Classification:Ambiguous
Severity Score:1.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- ADVERSE REACTIONS
- Cardiac Toxicity
- Acute development or exacerbation of congestive heart failure and new onset of decreased left ventricular ejection fraction have occurred during VELCADE therapy, including reports in patients with no risk factors for decreased left ventricular ejection fraction. Patients with risk factors for, or existing heart disease should be frequently monitored. In the relapsed multiple myeloma study of VELCADE vs dexamethasone, the incidence of any treatment-related cardiac disorder was 8% and 5% in the VELCADE and dexamethasone groups, respectively. The incidence of adverse reactions suggestive of heart failure (acute pulmonary edema, pulmonary edema, cardiac failure, congestive cardiac failure, cardiogenic shock) was ≤1% for each individual reaction in the VELCADE group. In the dexamethasone group the incidence was ≤1% for cardiac failure and congestive cardiac failure; there were no reported reactions of acute pulmonary edema, pulmonary edema, or cardiogenic shock. There have been isolated cases of QT-interval prolongation in clinical studies; causality has not been established.
- PATIENT COUNSELING INFORMATION
- Studies in monkeys and dogs showed that intravenous bortezomib doses as low as two times the recommended clinical dose on a mg/m2 basis were associated with increases in heart rate, decreases in contractility, hypotension, and death. In dog studies, a slight increase in the corrected QT interval was observed at doses resulting in death. In monkeys, doses of 3.0 mg/m2 and greater (approximately twice the recommended clinical dose) resulted in hypotension starting at one hour postadministration, with progression to death in 12 to 14 hours following drug administration.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
30
24062
Other ADRs
67830
38313757
Odds Ratio = 0.705
Drug Property Information
ATC Code(s):
- L01XG01 - bortezomib
- L01XG -
- L01X - OTHER ANTINEOPLASTIC AGENTS
- L01 - ANTINEOPLASTIC AGENTS
- L - ANTINEOPLASTIC AND IMMUNOMODULATING AGENTS
Active Ingredient:bortezomib
Active Ingredient UNII:69G8BD63PP
Drugbank ID:DB00188
PubChem Compound:387447
CTD ID:D000069286
PharmGKB:PA10252
CAS Number:179324-69-7
Dosage Form(s):injection, powder, lyophilized, for solution
Route(s) Of Administrator:intravenous; subcutaneous
Daily Dose:
Chemical Structure: 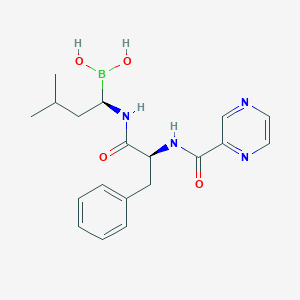

SMILE Code:
CC(C)C[C@H](NC(=O)[C@H](CC1=CC=CC=C1)NC(=O)C1=CN=CC=N1)B(O)O
CC(C)C[C@H](NC(=O)[C@H](CC1=CC=CC=C1)NC(=O)C1=CN=CC=N1)B(O)O
Reference
1: Phase 1 study of belinostat (PXD-101) and bortezomib (Velcade, PS-341) in patients with relapsed or refractory acute leukemia and myelodysplastic syndrome.
[Holkova Beata,Shafer Danielle,Yazbeck Victor,Dave Sandeep,Bose Prithviraj,Tombes Mary Beth,Shrader Ellen,Wan Wen,Bandyopadhyay Dipankar,Weir Caryn,Collins Elizabeth B,Garnett Amanda,Kmieciak Maciej,Roberts John D,Garcia-Manero Guillermo,Grant Steven]Leuk Lymphoma,2021 May;62(5):1187-1194. PMID: 33356689
2: Quisinostat, bortezomib, and dexamethasone combination therapy for relapsed multiple myeloma.
[Moreau Philippe,Facon Thierry,Touzeau Cyrille,Benboubker Lotfi,Delain Martine,Badamo-Dotzis Julie,Phelps Charles,Doty Christopher,Smit Hans,Fourneau Nele,Forslund Ann,Hellemans Peter,Leleu Xavier]Leuk Lymphoma,2016 Jul;57(7):1546-59. PMID: 26758913
3: Selective use of vandetanib in the treatment of thyroid cancer.
[Fallahi Poupak,Di Bari Flavia,Ferrari Silvia Martina,Spisni Roberto,Materazzi Gabriele,Miccoli Paolo,Benvenga Salvatore,Antonelli Alessandro]Drug Des Devel Ther,2015 Jul 3;9:3459-70. PMID: 26170630
4: Effect of levofloxacin prophylaxis for prevention of severe infections in multiple myeloma patients receiving bortezomib-containing regimens.
[Jung Sung-Hoon,Kang Seung-Ji,Jang Hee-Chang,Ahn Jae-Sook,Yang Deok-Hwan,Lee Seung-Shin,Kim Yeo-Kyeoung,Kim Hyeoung-Joon,Lee Je-Jung]Int J Hematol,2014 Nov;100(5):473-7. PMID: 25212681
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.