Search for drugs:
Typing the drug name to query
DROPERIDOL
DIR Classification
Classification:Most-DIQT concern
Severity Score:5.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- BOXED WARNING
- Cases of QT prolongation and/or torsade de pointes have been reported in patients receiving droperidol at doses at or below recommended doses. Some cases have occurred in patients with no known risk factors for QT prolongation and some cases have been fatal.
- Due to its potential for serious proarrhythmic effects and death, droperidol should be reserved for use in the treatment of patients who fail to show an acceptable response to other adequate treatments, either because of insufficient effectiveness or the inability to achieve an effective dose due to intolerable adverse effects from those drugs (see CONTRAINDICATIONS, WARNINGS, PRECAUTIONS, and ADVERSE REACTIONS).
- Cases of QT prolongation and serious arrhythmias (e.g., torsade de pointes) have been reported in patients treated with droperidol. Based on these reports, all patients should undergo a 12-lead ECG prior to administration of droperidol to determine if a prolonged QT interval (i.e., QTc greater than 440 msec for males or 450 msec for females) is present. If there is a prolonged QT interval, droperidol should NOT be administered. For patients in whom the potential benefit of droperidol treatment is felt to outweigh the risks of potentially serious arrhythmias, ECG monitoring should be performed prior to treatment and continued for 2-3 hours after completing treatment to monitor for arrhythmias.
- Droperidol is contraindicated in patients with known or suspected QT prolongation, including patients with congenital long QT syndrome.
- Droperidol should be administered with extreme caution to patients who may be at risk for development of prolonged QT syndrome (e.g., congestive heart failure, bradycardia, use of a diuretic, cardiac hypertrophy, hypokalemia, hypomagnesemia, or administration of other drugs known to increase the QT interval). Other risk factors may include age over 65 years, alcohol abuse, and use of agents such as benzodiazepines, volatile anesthetics, and IV opiates. Droperidol should be initiated at a low dose and adjusted upward, with caution, as needed to achieve the desired effect.
- WARNINGS
- Droperidol should be administered with extreme caution in the presence of risk factors for development of prolonged QT syndrome, such as: 1) clinically significant bradycardia (less than 50 bpm), 2) any clinically significant cardiac disease, 3) treatment with Class I and Class III antiarrhythmics, 4) treatment with monoamine oxidase inhibitors (MAOI's), 5) concomitant treatment with other drug products known to prolong the QT interval (see PRECAUTIONS, DRUG INTERACTIONS), and 6) electrolyte imbalance, in particular hypokalemia and hypomagnesemia, or concomitant treatment with drugs (e.g., diuretics) that may cause electrolyte imbalance.
- [Effects on Cardiac Conduction:]
- A dose-dependent prolongation of the QT interval was observed within 10 minutes of droperidol administration in a study of 40 patients without known cardiac disease who underwent extracranial head and neck surgery. Significant QT prolongation was observed at all three dose levels evaluated, with 0.1, 0.175, and 0.25 mg/kg associated with prolongation of median QTc by 37,44, and 59 msec, respectively.
- Cases of QT prolongation and serious arrhythmias (e.g., torsade de pointes, ventricular arrhythmias, cardiac arrest, and death) have been observed during post-marketing treatment with droperidol. Some cases have occurred in patients with no known risk factors and at doses at or below recommended doses. There has been at least one case of nonfatal torsade de pointes confirmed by rechallenge.
- Based on these reports, all patients should undergo a 12-lead ECG prior to administration of droperidol to determine if a prolonged QT interval (i.e., QTc greater than 440 msec for males or 450 msec for females) is present. If there is a prolonged QT interval, droperidol should NOT be administered. For patients in whom the potential benefit of droperidol treatment is felt to outweigh the risks of potentially serious arrhythmias, ECG monitoring should be performed prior to treatment and continued for 2-3 hours after completing treatment to monitor for arrhythmias.
- PRECAUTIONS
- Drug Interactions
- Potentially Arrhythmogenic Agents:
- Any drug known to have the potential to prolong the QT interval should not be used together with droperidol. Possible pharmacodynamic interactions can occur between droperidol and potentially arrhythmogenic agents such as class I or III antiarrhythmics, antihistamines that prolong the QT interval, antimalarials, calcium channel blockers, neuroleptics that prolong the QT interval, and antidepressants.
- Caution should be used when patients are taking concomitant drugs known to induce hypokalemia or hypomagnesemia as they may precipitate QT prolongation and interact with droperidol. These would include diuretics, laxatives and supraphysiological use of steroid hormones with mineralocorticoid potential.
- CONTRAINDICATIONS
- Droperidol is contraindicated in patients with known or suspected QT prolongation (i.e., QTc interval greater than 440 msec for males or 450 msec for females). This would include patients with congenital long QT syndrome.
- OVERDOSAGE
- Manifestations:
- The manifestations of droperidol overdosage are an extension of its pharmacologic actions and may include QT prolongation and serious arrhythmias (e.g., torsade de pointes) (see BOXED WARNING, WARNINGS, and PRECAUTIONS).
- ADVERSE REACTIONS
- QT interval prolongation, torsade de pointes, cardiac arrest, and ventricular tachycardia have been reported in patients treated with droperidol. Some of these cases were associated with death. Some cases occurred in patients with no known risk factors, and some were associated with droperidol doses at or below recommended doses.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
3
24089
Other ADRs
282
38381305
Odds Ratio = 16.951
Drug Property Information
ATC Code(s):
- N05AD08 - droperidol
- N05AD - Butyrophenone derivatives
- N05A - ANTIPSYCHOTICS
- N05 - PSYCHOLEPTICS
- N - NERVOUS SYSTEM
Active Ingredient:DROPERIDOL
Active Ingredient UNII:O9U0F09D5X
Drugbank ID:DB00450
PubChem Compound:3168
CTD ID:D004329
PharmGKB:PA449422
CAS Number:548-73-2
Dosage Form(s):injection, solution
Route(s) Of Administrator:intramuscular; intravenous
Daily Dose:
- 2.5 mg/day N05AD08
Chemical Structure: 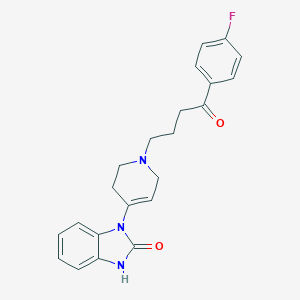

SMILE Code:
FC1=CC=C(C=C1)C(=O)CCCN1CCC(=CC1)N1C(=O)NC2=CC=CC=C12
FC1=CC=C(C=C1)C(=O)CCCN1CCC(=CC1)N1C(=O)NC2=CC=CC=C12
Reference
1: Comment on "The Incidence of QT Prolongation and Torsades des Pointes in Patients Receiving Droperidol in an Urban Emergency Department".
[DiSalvo Philip C,Backus Timothy C,Chiang William K]West J Emerg Med,2021 Jan 11;22(2):394-395. PMID: 33856328
2: Safety and efficacy of pharmacologic agents used for rapid tranquilization of emergency department patients with acute agitation or excited delirium.
[Kim Hong K,Leonard James B,Corwell Brian N,Connors Nicholas J]Expert Opin Drug Saf,2021 Feb;20(2):123-138. PMID: 33327811
3: Quetiapine and other antipsychotics combined with opioids in legal autopsy cases: A random finding or cause of fatal outcome?
[Andersen Freja Drost,Simonsen Ulf,Andersen Charlotte Uggerhøj]Basic Clin Pharmacol Toxicol,2021 Jan;128(1):66-79. PMID: 33245632
4: Arrhythmias related to antipsychotics and antidepressants: an analysis of the summaries of product characteristics of original products approved in Germany.
[Elsayed Mohamed,Abdel-Kahaar Emaad,Gahr Maximilian,Connemann Bernhard J,Denkinger Michael,Schönfeldt-Lecuona Carlos]Eur J Clin Pharmacol,2021 May;77(5):767-775. PMID: 33230596
5: Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.
[Weibel Stephanie,Rücker Gerta,Eberhart Leopold Hj,Pace Nathan L,Hartl Hannah M,Jordan Olivia L,Mayer Debora,Riemer Manuel,Schaefer Maximilian S,Raj Diana,Backhaus Insa,Helf Antonia,Schlesinger Tobias,Kienbaum Peter,Kranke Peter]Cochrane Database Syst Rev,2020 Oct 19;10:CD012859. PMID: 33075160
6: The Incidence of QT Prolongation and Torsades des Pointes in Patients Receiving Droperidol in an Urban Emergency Department.
[Cole Jon B,Lee Samantha C,Martel Marc L,Smith Stephen W,Biros Michelle H,Miner James R]West J Emerg Med,2020 Jul 2;21(4):728-736. PMID: 32726229
7: Perianesthesia Implications and Considerations for Drug-Induced QT Interval Prolongation.
[Aroke Edwin N,Nkemazeh Rolland Z]J Perianesth Nurs,2020 Apr;35(2):104-111. PMID: 31955897
8: Effectiveness and safety of droperidol in a United States emergency department.
[Gaw Charlene M,Cabrera Daniel,Bellolio Fernanda,Mattson Alicia E,Lohse Christine M,Jeffery Molly M]Am J Emerg Med,2020 Jul;38(7):1310-1314. PMID: 31831345
9: Prevalence of high-risk drug-drug interactions in paediatric inpatients: a retrospective, single-centre cohort analysis.
[Dieu Ly Wen,Leuppi-Taegtmeyer Anne B,van den Anker John,Trachsel Daniel,Gotta Verena]Swiss Med Wkly,2019 Aug 18;149:w20103. PMID: 31422575
10: Antipsychotics for patients with Wolff-Parkinson-White Syndrome.
[Nutting Sean,Martin Christopher,Prensner Richard,Francis Andrew,Bellon Alfredo]Clin Schizophr Relat Psychoses,2019 Jan 3. PMID: 30649911
11: Evidence-based review and appraisal of the use of droperidol in the emergency department.
[Lai Pei-Chun,Huang Yen-Ta]Ci Ji Yi Xue Za Zhi,Jan-Mar 2018;30(1):1-4. PMID: 29643708
12: Specific prediction of clinical QT prolongation by kinetic image cytometry in human stem cell derived cardiomyocytes.
[Pfeiffer Emily R,Vega Raquel,McDonough Patrick M,Price Jeffrey H,Whittaker Ross]J Pharmacol Toxicol Methods,Sep-Oct 2016;81:263-73. PMID: 27095424
13: [The Interaction of Low-dose Droperidol, Propofol, and Sevoflurane on QTc Prolongation].
[Toyoda Tomomi,Terao Yoshiaki,Oji Makito,Okada Mai,Araki Hiroko,Fukusaki Makoto]Masui,2015 Jun;64(6):580-5. PMID: 26437544
14: The Safety and Effectiveness of Droperidol for Sedation of Acute Behavioral Disturbance in the Emergency Department.
[Calver Leonie,Page Colin B,Downes Michael A,Chan Betty,Kinnear Frances,Wheatley Luke,Spain David,Isbister Geoffrey Kennedy]Ann Emerg Med,2015 Sep;66(3):230-238.e1. PMID: 25890395
15: Effects of droperidol and ondansetron on dispersion of ventricular repolarization: A randomized double-blind clinical study in anesthetized adult patients.
[Agámez Medina G L,González-Arévalo A,Gómez-Arnau J I,García del Valle S,Rubio J A,Esteban E,Pérez E]Rev Esp Anestesiol Reanim,2015 Nov;62(9):495-501. PMID: 25887095
16: American Academy of Emergency Medicine Position Statement: Safety of Droperidol Use in the Emergency Department.
[Perkins Jack,Ho Jeffrey D,Vilke Gary M,DeMers Gerard]J Emerg Med,2015 Jul;49(1):91-7. PMID: 25837231
17: Droperidol for the treatment of acute migraine headaches.
[Thomas Michael C,Musselman Megan E,Shewmaker Justin]Ann Pharmacother,2015 Feb;49(2):233-40. PMID: 25416184
18: Small doses of droperidol do not present relevant torsadogenic actions: a double-blind, ondansetron-controlled study.
[Tracz Krzysztof,Owczuk Radosław]Br J Clin Pharmacol,2015 Apr;79(4):669-76. PMID: 25293524
19: Dopamine antagonists for nausea and vomiting: special considerations.
[Welliver Mark]Gastroenterol Nurs,Sep-Oct 2014;37(5):361-4. PMID: 25271829
20: Droperidol transiently prolongs the QT interval in children undergoing single ventricle palliation.
[Scott John P,Stuth Eckehard A E,Stucke Astrid G,Cava Joseph R,Berens Richard J]Pediatr Cardiol,2015 Jan;36(1):196-204. PMID: 25087057
21: Comparison of droperidol and haloperidol for use by paramedics: assessment of safety and effectiveness.
[Macht Marlow,Mull Ashley C,McVaney Kevin E,Caruso Emily H,Johnston J Bill,Gaither Joshua B,Shupp Aaron M,Marquez Kevin D,Haukoos Jason S,Colwell Christopher B]Prehosp Emerg Care,Jul-Sep 2014;18(3):375-80. PMID: 24460451
22: Impact of anaesthetic drugs and adjuvants on ECG markers of torsadogenicity.
[Staikou C,Stamelos M,Stavroulakis E]Br J Anaesth,2014 Feb;112(2):217-30. PMID: 24305646
23: High dose droperidol and QT prolongation: analysis of continuous 12-lead recordings.
[Calver Leonie,Isbister Geoffrey K]Br J Clin Pharmacol,2014 May;77(5):880-6. PMID: 24168079
24: Parenteral sedation of elderly patients with acute behavioral disturbance in the ED.
[Calver Leonie,Isbister Geoffrey K]Am J Emerg Med,2013 Jun;31(6):970-3. PMID: 23685060
25: Does low-dose droperidol increase the risk of polymorphic ventricular tachycardia or death in the surgical patient?
[Nuttall Gregory A,Malone Ann M,Michels Carrie A,Trudell Laurie C,Renk Tricia D,Marienau Mary E Shirk,Oliver William C,Ackerman Michael J]Anesthesiology,2013 Feb;118(2):382-6. PMID: 23291623
26: The clinical significance of QT interval prolongation in anesthesia and pain management: what you should and should not worry about.
[Spevak Christopher,Hamsher Carlyle,Brown Carlton Q,Wedam Erich F,Haigney Mark C]Pain Med,2012 Aug;13(8):1072-80. PMID: 22680349
27: Survey of methadone-drug interactions among patients of methadone maintenance treatment program in Taiwan.
[Lee Hsin-Ya,Li Jih-Heng,Wu Li-Tzy,Wu Jin-Song,Yen Cheng-Fang,Tang Hsin-Pei]Subst Abuse Treat Prev Policy,2012 Mar 20;7:11. PMID: 22429858
28: [Low-dose droperidol in children: rescue therapy for persistent postoperative nausea and vomiting].
[Schroeter E,Schmitz A,Haas T,Weiss M,Gerber A C]Anaesthesist,2012 Jan;61(1):30-4. PMID: 22234576
29: Effect of intravenous ondansetron on QT interval prolongation in patients with cardiovascular disease and additional risk factors for torsades: a prospective, observational study.
[Hafermann Matthew J,Namdar Rocsanna,Seibold Gretchen E,Page Robert Lee]Drug Healthc Patient Saf,2011;3:53-8. PMID: 22046106
30: Randomized controlled trial of intramuscular droperidol versus midazolam for violence and acute behavioral disturbance: the DORM study.
[Isbister Geoffrey K,Calver Leonie A,Page Colin B,Stokes Barrie,Bryant Jenni L,Downes Michael A]Ann Emerg Med,2010 Oct;56(4):392-401.e1. PMID: 20868907
31: The effects of droperidol and ondansetron on dispersion of myocardial repolarization in children.
[Mehta Disha,Sanatani Shubhayan,Whyte Simon D]Paediatr Anaesth,2010 Oct;20(10):905-12. PMID: 20849495
32: Iatrogenic QT Abnormalities and Fatal Arrhythmias: Mechanisms and Clinical Significance.
[Cubeddu Luigi X]Curr Cardiol Rev,2009 Aug;5(3):166-76. PMID: 20676275
33: QT alterations in psychopharmacology: proven candidates and suspects.
[Alvarez Paulino Antonio,Pahissa Jaime]Curr Drug Saf,2010 Jan;5(1):97-104. PMID: 20210726
34: Ventricular tachycardia after ondansetron administration in a child with undiagnosed long QT syndrome.
[McKechnie Kyle,Froese Alison]Can J Anaesth,2010 May;57(5):453-7. PMID: 20204717
35: Sevoflurane causes greater QTc interval prolongation in elderly patients than in younger patients.
[Nakao Shinichi,Hatano Kiyohiko,Sumi Chisato,Masuzawa Munehiro,Sakamoto Sachiyo,Ikeda Sakahiro,Shingu Koh]Anesth Analg,2010 Mar 1;110(3):775-9. PMID: 20185656
36: Antiemetic therapy for nausea and vomiting in the emergency department.
[Patanwala Asad E,Amini Richard,Hays Daniel P,Rosen Peter]J Emerg Med,2010 Sep;39(3):330-6. PMID: 20022195
37: Torsade de Pointes after administration of droperidol for nausea and vomiting.
[Choo Esther K,Weber Frank S,Schmidt Terri A]Prehosp Emerg Care,Apr-Jun 2009;13(2):261-5. PMID: 19291568
38: Droperidol and ondansetron-induced QT interval prolongation: a clinical drug interaction study.
[Charbit Beny,Alvarez Jean Claude,Dasque Eric,Abe Emuri,Démolis Jean Louis,Funck-Brentano Christian]Anesthesiology,2008 Aug;109(2):206-12. PMID: 18648229
39: The prophylactic effect of haloperidol plus dexamethasone on postoperative nausea and vomiting in patients undergoing laparoscopically assisted vaginal hysterectomy.
[Chu Chin-Chen,Shieh Ja-Ping,Tzeng Jann-Inn,Chen Jen-Yin,Lee Yi,Ho Shung-Tai,Wang Jhi-Joung]Anesth Analg,2008 May;106(5):1402-6, table of contents. PMID: 18420851
40: Inhibition of the HERG channel by droperidol depends on channel gating and involves the S6 residue F656.
[Luo Tao,Luo Ailin,Liu Miu,Liu Xianyi]Anesth Analg,2008 Apr;106(4):1161-70, table of contents. PMID: 18349188
41: Does low-dose droperidol administration increase the risk of drug-induced QT prolongation and torsade de pointes in the general surgical population?
[Nuttall Gregory A,Eckerman Karen M,Jacob Kelly A,Pawlaski Erin M,Wigersma Susan K,Marienau Mary E Shirk,Oliver William C,Narr Bradly J,Ackerman Michael J]Anesthesiology,2007 Oct;107(4):531-6. PMID: 17893447
42: State dependent dissociation of HERG channel inhibitors.
[Stork D,Timin E N,Berjukow S,Huber C,Hohaus A,Auer M,Hering S]Br J Pharmacol,2007 Aug;151(8):1368-76. PMID: 17592502
43: The additive interactions between ondansetron and droperidol for preventing postoperative nausea and vomiting.
[Chan Matthew T V,Choi Kai C,Gin Tony,Chui Po Tong,Short Timothy G,Yuen Pong Mo,Poon Amy H Y,Apfel Christian C,Gan Tong J]Anesth Analg,2006 Nov;103(5):1155-62. PMID: 17056948
44: Safety pharmacology assessment of drug-induced QT-prolongation in dogs with reduced repolarization reserve.
[Vormberge Thomas,Hoffmann Michael,Himmel Herbert]J Pharmacol Toxicol Methods,Sep-Oct 2006;54(2):130-40. PMID: 16757186
45: Anaesthetics and the rate corrected interval: learning from droperidol?
[Shipton Edward A]Curr Opin Anaesthesiol,2005 Aug;18(4):419-23. PMID: 16534268
46: Effect of low-dose droperidol on the QT interval during and after general anesthesia: a placebo-controlled study.
[White Paul F,Song Dajun,Abrao Joao,Klein Kevin W,Navarette Bryan]Anesthesiology,2005 Jun;102(6):1101-5. PMID: 15915020
47: Atypical antipsychotics: from potassium channels to torsade de pointes and sudden death.
[Titier Karine,Girodet Pierre-Olivier,Verdoux Hélène,Molimard Mathieu,Bégaud Bernard,Haverkamp Wilhelm,Lader Malcolm,Moore Nicholas]Drug Saf,2005;28(1):35-51. PMID: 15649104
48: Droperidol for perioperative sedation causes a transient prolongation of the QTc time in children under volatile anesthesia.
[Stuth Eckehard A E,Stucke Astrid G,Cava Joseph R,Hoffman George M,Berens Richard J]Paediatr Anaesth,2004 Oct;14(10):831-7. PMID: 15385011
49: Safety of droperidol in behavioural emergencies.
[Shale John H,Shale Christopher M,Mastin William D]Expert Opin Drug Saf,2004 Jul;3(4):369-78. PMID: 15268653
50: The relevance of prolonged QTc measurement to pediatric psychopharmacology.
[Labellarte Michael J,Crosson Jane E,Riddle Mark A]J Am Acad Child Adolesc Psychiatry,2003 Jun;42(6):642-50. PMID: 12921471
51: Droperidol in the emergency department: is it safe?
[Richards John R,Schneir Aaron B]J Emerg Med,2003 May;24(4):441-7. PMID: 12745049
52: Droperidol, QT prolongation, and sudden death: what is the evidence?
[Kao Louise W,Kirk Mark A,Evers Stephanee J,Rosenfeld Stephen H]Ann Emerg Med,2003 Apr;41(4):546-58. PMID: 12658255
53: Droperidol: should the black box be light gray?
[Dershwitz Mark]J Clin Anesth,2002 Dec;14(8):598-603. PMID: 12565120
54: Acute migraine treatment with droperidol: A randomized, double-blind, placebo-controlled trial.
[Silberstein S D,Young W B,Mendizabal J E,Rothrock J F,Alam A S]Neurology,2003 Jan 28;60(2):315-21. PMID: 12552051
55: Management of the violent patient.
[Brice Jane H,Pirrallo Ronald G,Racht Edward,Zachariah Brian S,Krohmer Jon]Prehosp Emerg Care,Jan-Mar 2003;7(1):48-55. PMID: 12540143
56: The effects of the FDA warning on the use of droperidol by u.s. Emergency physicians.
[Richards John R,Weiss Steven J,Bretz Stephen W,Schneir Aaron B,Rinetti Dauna,Derlet Robert W]Cal J Emerg Med,2003 Jan;4(1):3-9. PMID: 20852711
57: Antipsychotic-related QTc prolongation, torsade de pointes and sudden death.
[Haddad Peter M,Anderson Ian M]Drugs,2002;62(11):1649-71. PMID: 12109926
58: Antipsychotic drugs: prolonged QTc interval, torsade de pointes, and sudden death.
[Glassman A H,Bigger J T]Am J Psychiatry,2001 Nov;158(11):1774-82. PMID: 11691681
59: Drug interactions with cisapride: clinical implications.
[Michalets E L,Williams C R]Clin Pharmacokinet,2000 Jul;39(1):49-75. PMID: 10926350
60: Droperidol lengthens cardiac repolarization due to block of the rapid component of the delayed rectifier potassium current.
[Drolet B,Zhang S,Deschênes D,Rail J,Nadeau S,Zhou Z,January C T,Turgeon J]J Cardiovasc Electrophysiol,1999 Dec;10(12):1597-604. PMID: 10636190
61: Torsade de pointes resulting from the addition of droperidol to an existing cytochrome P450 drug interaction.
[Michalets E L,Smith L K,Van Tassel E D]Ann Pharmacother,Jul-Aug 1998;32(7-8):761-5. PMID: 9681092
62: Torsade de pointes complicating the treatment of bleeding esophageal varices: association with neuroleptics, vasopressin, and electrolyte imbalance.
[Faigel D O,Metz D C,Kochman M L]Am J Gastroenterol,1995 May;90(5):822-4. PMID: 7733096
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.