Search for drugs:
Typing the drug name to query
SALMETEROL XINAFOATE
DIR Classification
Classification:Moderate-DIQT concern
Severity Score:3.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- WARNINGS AND PRECAUTIONS
- Cardiovascular and Central Nervous System Effects
- Salmeterol can produce a clinically significant cardiovascular effect in some patients as measured by pulse rate, blood pressure, and/or symptoms. Although such effects are uncommon after administration of salmeterol at recommended doses, if they occur, the drug may need to be discontinued. In addition, beta-agonists have been reported to produce electrocardiogram (ECG) changes, such as flattening of the T wave, prolongation of the QTc interval, and ST segment depression. The clinical significance of these findings is unknown. Large doses of inhaled or oral salmeterol (12 to 20 times the recommended dose) have been associated with clinically significant prolongation of the QTc interval, which has the potential for producing ventricular arrhythmias. Fatalities have been reported in association with excessive use of inhaled sympathomimetic drugs.
- DRUG INTERACTIONS
- In a drug interaction trial in 20 healthy subjects, coadministration of inhaled salmeterol (50 mcg twice daily) and oral ketoconazole (400 mg once daily) for 7 days resulted in greater systemic exposure to salmeterol (AUC increased 16-fold and Cmax increased 1.4-fold). Three (3) subjects were withdrawn due to beta2-agonist side effects (2 with prolonged QTc and 1 with palpitations and sinus tachycardia). Although there was no statistical effect on the mean QTc, coadministration of salmeterol and ketoconazole was associated with more frequent increases in QTc duration compared with salmeterol and placebo administration.
- OVERDOSAGE
- Overdosage with SEREVENT DISKUS can lead to clinically significant prolongation of the QTc interval, which can produce ventricular arrhythmias.
- CLINICAL PHARMACOLOGY
- Drug Interaction Studies
- Inhibitors of Cytochrome P450 3A4: Ketoconazole: In a placebo-controlled crossover drug interaction trial in 20 healthy male and female subjects, coadministration of salmeterol (50 mcg twice daily) and the strong CYP3A4 inhibitor ketoconazole (400 mg once daily) for 7 days resulted in a significant increase in plasma salmeterol exposure as determined by a 16-fold increase in AUC (ratio with and without ketoconazole 15.76 [90% CI: 10.66, 23.31]) mainly due to increased bioavailability of the swallowed portion of the dose. Peak plasma salmeterol concentrations were increased by 1.4-fold (90% CI: 1.23, 1.68). Three (3) out of 20 subjects (15%) were withdrawn from salmeterol and ketoconazole coadministration due to beta-agonist鈥搈ediated systemic effects (2 with QTc prolongation and 1 with palpitations and sinus tachycardia). Coadministration of salmeterol and ketoconazole did not result in a clinically significant effect on mean heart rate, mean blood potassium, or mean blood glucose. Although there was no statistical effect on the mean QTc, coadministration of salmeterol and ketoconazole was associated with more frequent increases in QTc duration compared with salmeterol and placebo administration.
- Erythromycin: In a repeat-dose trial in 13 healthy subjects, concomitant administration of erythromycin (a moderate CYP3A4 inhibitor) and salmeterol inhalation aerosol resulted in a 40% increase in salmeterol Cmax at steady state (ratio with and without erythromycin 1.4 [90% CI: 0.96, 2.03], P = 0.12), a 3.6-beat/min increase in heart rate ([95% CI: 0.19, 7.03], P<0.04), a 5.8-msec increase in QTc interval ([95% CI: -6.14, 17.77], P = 0.34), and no change in plasma potassium.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
0
24092
Other ADRs
5198
38376389
Odds Ratio = 0.0
Drug Property Information
ATC Code(s):
- R03AC12 - salmeterol xinafoate
- R03AC - Selective beta-2-adrenoreceptor agonists
- R03A - "ADRENERGICS, INHALANTS"
- R03 - DRUGS FOR OBSTRUCTIVE AIRWAY DISEASES
- R - RESPIRATORY SYSTEM
Active Ingredient:SALMETEROL XINAFOATE
Active Ingredient UNII:6EW8Q962A5
Drugbank ID:DB00938
PubChem Compound:5152
CTD ID:D000068299
PharmGKB:PA451300
CAS Number:89365-50-4
Dosage Form(s):powder, metered
Route(s) Of Administrator:oral; respiratory (inhalation)
Daily Dose:
- 0.1 mg/day R03AC12
Chemical Structure: 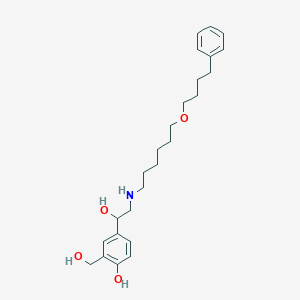

SMILE Code:
OCC1=C(O)C=CC(=C1)C(O)CNCCCCCCOCCCCC1=CC=CC=C1
OCC1=C(O)C=CC(=C1)C(O)CNCCCCCCOCCCCC1=CC=CC=C1
Reference
N/A
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.