Search for drugs:
Typing the drug name to query
DESLORATADINE
DIR Classification
Classification:Ambiguous
Severity Score:1.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- ADVERSE REACTIONS
- Clinical Trials Experience
- There were no clinically meaningful changes in any electrocardiographic parameter, including the QTc interval. Only one of the 246 pediatric subjects receiving Desloratadine Oral Solution in the clinical trials discontinued treatment because of an adverse event.
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Effects on QTc: Single daily doses of 45 mg were given to normal male and female volunteers for 10 days. All ECGs obtained in this study were manually read in a blinded fashion by a cardiologist. In Desloratadine-treated subjects, there was an increase in mean heart rate of 9.2 bpm relative to placebo. The QT interval was corrected for heart rate (QTc) by both the Bazett and Fridericia methods. Using the QTc (Bazett) there was a mean increase of 8.1 msec in Desloratadine-treated subjects relative to placebo. Using QTc (Fridericia) there was a mean increase of 0.4 msec in Desloratadine-treated subjects relative to placebo. No clinically relevant adverse events were reported.
- [Pharmacokinetics]
- Drug Interactions: In two controlled crossover clinical pharmacology studies in healthy male (n=12 in each study) and female (n=12 in each study) volunteers, desloratadine 7.5 mg (1.5 times the daily dose) once daily was coadministered with erythromycin 500 mg every 8 hours or ketoconazole 200 mg every 12 hours for 10 days. In three separate controlled, parallel group clinical pharmacology studies, desloratadine at the clinical dose of 5 mg has been coadministered with azithromycin 500 mg followed by 250 mg once daily for 4 days (n=18) or with fluoxetine 20 mg once daily for 7 days after a 23-day pretreatment period with fluoxetine (n=18) or with cimetidine 600 mg every 12 hours for 14 days (n=18) under steady-state conditions to normal healthy male and female volunteers. Although increased plasma concentrations (Cmax and AUC0-24 hrs) of desloratadine and 3-hydroxydesloratadine were observed (see TABLE 2), there were no clinically relevant changes in the safety profile of desloratadine, as assessed by electrocardiographic parameters (including the corrected QT interval), clinical laboratory tests, vital signs, and adverse events.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
37
24055
Other ADRs
5667
38375920
Odds Ratio = 10.417
Drug Property Information
ATC Code(s):
- R06AX27 - desloratadine
- R06AX - Other antihistamines for systemic use
- R06A - ANTIHISTAMINES FOR SYSTEMIC USE
- R06 - ANTIHISTAMINES FOR SYSTEMIC USE
- R - RESPIRATORY SYSTEM
Active Ingredient:DESLORATADINE
Active Ingredient UNII:FVF865388R
Drugbank ID:DB00967
PubChem Compound:124087
CTD ID:C121345
PharmGKB:PA164776964
CAS Number:100643-71-8
Dosage Form(s):tablet
Route(s) Of Administrator:oral
Daily Dose:
- 5.0 mg/day R06AX27
Chemical Structure: 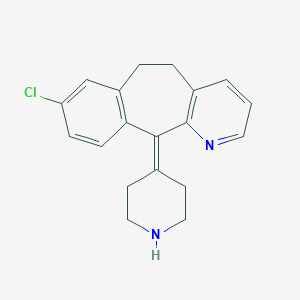

SMILE Code:
ClC1=CC2=C(C=C1)C(=C1CCNCC1)C1=C(CC2)C=CC=N1
ClC1=CC2=C(C=C1)C(=C1CCNCC1)C1=C(CC2)C=CC=N1
Reference
1: Association of H1-antihistamines with torsade de pointes: a pharmacovigilance study of the food and drug administration adverse event reporting system.
[Ali Zahid,Ismail Mohammad,Khan Fahadullah,Sajid Hira]Expert Opin Drug Saf,2021 Jan;20(1):101-107. PMID: 33141610
2: {'#text': 'Cardiac safety of second-generation H -antihistamines when updosed in chronic spontaneous urticaria.', 'sub': '1'}
[Cataldi Mauro,Maurer Marcus,Taglialatela Maurizio,Church Martin K]Clin Exp Allergy,2019 Dec;49(12):1615-1623. PMID: 31519068
3: Evidence for the hERG Liability of Antihistamines, Antipsychotics, and Anti-Infective Agents: A Systematic Literature Review From the ARITMO Project.
[Hazell Lorna,Raschi Emanuel,De Ponti Fabrizio,Thomas Simon H L,Salvo Francesco,Ahlberg Helgee Ernst,Boyer Scott,Sturkenboom Miriam,Shakir Saad]J Clin Pharmacol,2017 May;57(5):558-572. PMID: 28019033
4: Pro-arrhythmic potential of oral antihistamines (H1): combining adverse event reports with drug utilization data across Europe.
[Poluzzi Elisabetta,Raschi Emanuel,Godman Brian,Koci Ariola,Moretti Ugo,Kalaba Marija,Wettermark Bjorn,Sturkenboom Miriam,De Ponti Fabrizio]PLoS One,2015 Mar 18;10(3):e0119551. PMID: 25785934
5: Cetirizine and loratadine: minimal risk of QT prolongation.
Prescrire Int,2010 Feb;19(105):26-8. PMID: 20455340
6: Comparative pharmacology of guinea pig cardiac myocyte and cloned hERG (I(Kr)) channel.
[Davie Christina,Pierre-Valentin Jean,Pollard Chris,Standen Nick,Mitcheson John,Alexander Paul,Thong Bob]J Cardiovasc Electrophysiol,2004 Nov;15(11):1302-9. PMID: 15574182
7: Comparative effects of loratadine and terfenadine on cardiac K+ channels.
[Ducic I,Ko C M,Shuba Y,Morad M]J Cardiovasc Pharmacol,1997 Jul;30(1):42-54. PMID: 9268220
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.