Search for drugs:
Typing the drug name to query
IBRUTINIB
DIR Classification
Classification:Ambiguous
Severity Score:1.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Cardiac Electrophysiology
- At a single dose 3 times the maximum recommended dose (1680 mg), IMBRUVICA did not prolong the QT interval to any clinically relevant extent.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
15
24077
Other ADRs
126866
38254721
Odds Ratio = 0.188
Drug Property Information
ATC Code(s):
- L01EL01 - ibrutinib
- L01EL0 -
- L01EL -
- L01E -
- L01 - ANTINEOPLASTIC AGENTS
- L - ANTINEOPLASTIC AND IMMUNOMODULATING AGENTS
Active Ingredient:IBRUTINIB
Active Ingredient UNII:1X70OSD4VX
Drugbank ID:DB09053
PubChem Compound:24821094
CTD ID: C551803
PharmGKB:PA166121346
CAS Number:936563-96-1
Dosage Form(s):capsule; tablet, film coated
Route(s) Of Administrator:oral
Daily Dose:
Chemical Structure: 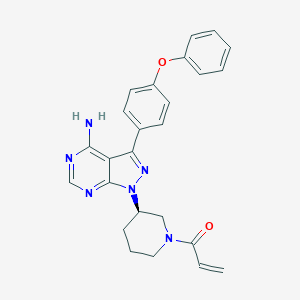

SMILE Code:
NC1=NC=NC2=C1C(=NN2[C@@H]1CCCN(C1)C(=O)C=C)C1=CC=C(OC2=CC=CC=C2)C=C1
NC1=NC=NC2=C1C(=NN2[C@@H]1CCCN(C1)C(=O)C=C)C1=CC=C(OC2=CC=CC=C2)C=C1
Reference
1: Anticancer drug-induced cardiac rhythm disorders: Current knowledge and basic underlying mechanisms.
[Alexandre Joachim,Moslehi Javid J,Bersell Kevin R,Funck-Brentano Christian,Roden Dan M,Salem Joe-Elie]Pharmacol Ther,2018 Sep;189:89-103. PMID: 29698683
2: Update on Cardiovascular Safety of Tyrosine Kinase Inhibitors: With a Special Focus on QT Interval, Left Ventricular Dysfunction and Overall Risk/Benefit.
[Shah Rashmi R,Morganroth Joel]Drug Saf,2015 Aug;38(8):693-710. PMID: 26008987
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.