Search for drugs:
Typing the drug name to query
DOFETILIDE
DIR Classification
Classification:Most-DIQT concern
Severity Score:4.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- WARNINGS
- Ventricular Arrhythmia
- Dofetilide can cause serious ventricular arrhythmias, primarily Torsade de Pointes (TdP) type ventricular tachycardia, a polymorphic ventricular tachycardia associated with QT interval prolongation. QT interval prolongation is directly related to dofetilide plasma concentration. Factors such as reduced creatinine clearance or certain dofetilide drug interactions will increase dofetilide plasma concentration. The risk of TdP can be reduced by controlling the plasma concentration through adjustment of the initial dofetilide dose according to creatinine clearance and by monitoring the ECG for excessive increases in the QT interval.
- Treatment with dofetilide must therefore be started only in patients placed for a minimum of three days in a facility that can provide electrocardiographic monitoring and in the presence of personnel trained in the management of serious ventricular arrhythmias. Calculation of the creatinine clearance for all patients must precede administration of the first dose of dofetilide. For detailed instructions regarding dose selection, see DOSAGE AND ADMINISTRATION.
- The risk of dofetilide induced ventricular arrhythmia was assessed in three ways in clinical studies: 1) by description of the QT interval and its relation to the dose and plasma concentration of dofetilide; 2) by observing the frequency of TdP in dofetilide-treated patients according to dose; 3) by observing the overall mortality rate in patients with atrial fibrillation and in patients with structural heart disease.
- Relation of QT Interval to Dose:
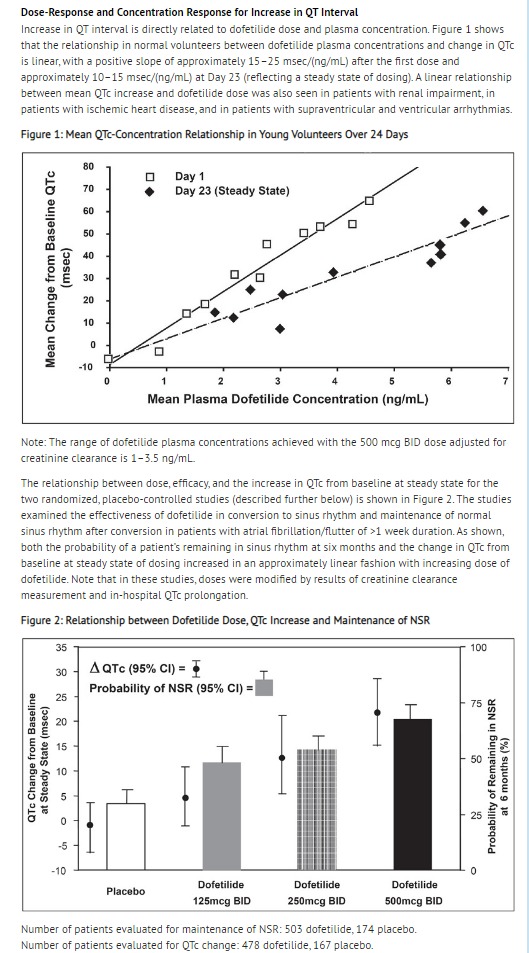
- The QT interval increases linearly with increasing dofetilide dose (see Figures 1 and 2 in CLINICAL PHARMACOLOGY and Dose-Response and Concentration Response for Increase in QT Interval).
- [Frequency of Torsade de Pointes]
- In the supraventricular arrhythmia population (patients with AF and other supraventricular arrhythmias), the overall incidence of Torsade de Pointes was 0.8%. The frequency of TdP by dose is shown in Table 4. There were no cases of TdP on placebo.
- [Drug-Drug Interactions]
- (see CONTRAINDICATIONS)
- Because there is a linear relationship between dofetilide plasma concentration and QTc, concomitant drugs that interfere with the metabolism or renal elimination of dofetilide may increase the risk of arrhythmia (Torsade de Pointes). Dofetilide is metabolized to a small degree by the CYP3A4 isoenzyme of the cytochrome P450 system and an inhibitor of this system could increase systemic dofetilide exposure. More important, dofetilide is eliminated by cationic renal secretion, and three inhibitors of this process have been shown to increase systemic dofetilide exposure. The magnitude of the effect on renal elimination by cimetidine, trimethoprim, and ketoconazole (all contraindicated concomitant uses with dofetilide) suggests that all renal cation transport inhibitors should be contraindicated.
- Hypokalemia and Potassium-Depleting Diuretics
- Hypokalemia or hypomagnesemia may occur with administration of potassium-depleting diuretics, increasing the potential for Torsade de Pointes. Potassium levels should be within the normal range prior to administration of dofetilide and maintained in the normal range during administration of dofetilide (see DOSAGE AND ADMINISTRATION).
- [Use with Drugs that Prolong QT Interval and Antiarrhythmic Agents]
- The use of dofetilide in conjunction with other drugs that prolong the QT interval has not been studied and is not recommended. Such drugs include phenothiazines, cisapride, bepridil, tricyclic antidepressants, certain oral macrolides, and certain fluoroquinolones. Class I or Class III antiarrhythmic agents should be withheld for at least three half-lives prior to dosing with dofetilide. In clinical trials, dofetilide was administered to patients previously treated with oral amiodarone only if serum amiodarone levels were below 0.3 mg/L or amiodarone had been withdrawn for at least three months.
- PRECAUTIONS
- Information for Patients
- Please refer patient to the Medication Guide.
- Prior to initiation of dofetilide therapy, the patient should be advised to read the Medication Guide and reread it each time therapy is renewed in case the patient's status has changed. The patient should be fully instructed on the need for compliance with the recommended dosing of dofetilide capsules and the potential for drug interactions, and the need for periodic monitoring of QTc and renal function to minimize the risk of serious abnormal rhythms.
- [Hydrochlorothiazide (HCTZ) Alone or in Combination with Triamterene]
- (see CONTRAINDICATIONS) Concomitant use of HCTZ alone or in combination with triamterene is contraindicated. HCTZ 50 mg QD or HCTZ/triamterene 50/100 mg QD was co-administered with dofetilide (500 mcg BID) for 5 days (following 2 days of diuretic use at half dose). In patients receiving HCTZ alone, dofetilide AUC increased by 27% and C max by 21%. However, the pharmacodynamic effect increased by 197% (QTc increase over time) and by 95% (maximum QTc increase). In patients receiving HCTZ in combination with triamterene, dofetilide AUC increased by 30% and C max by 16%. However, the pharmacodynamic effect increased by 190% (QTc increase over time) and by 84% (maximum QTc increase). The pharmacodynamic effects can be explained by a combination of the increase in dofetilide exposure and the reductions in serum potassium. In the DIAMOND trials, 1252 patients were treated with dofetilide and diuretics concomitantly, of whom 493 died compared to 508 deaths among the 1248 patients receiving placebo and diuretics. Of the 229 patients who had potassium depleting diuretics added to their concomitant medications in the DIAMOND trials, the patients on dofetilide had a non-significantly reduced relative risk for death of 0.68 (95% CI: 0.376, 1.230).
- [Other Drugs]
- Population pharmacokinetic analyses were conducted on plasma concentration data from 1445 patients in clinical trials to examine the effects of concomitant medications on clearance or volume of distribution of dofetilide. Concomitant medications were grouped as ACE inhibitors, oral anticoagulants, calcium channel blockers, beta blockers, cardiac glycosides, inducers of CYP3A4, substrates and inhibitors of CYP3A4, substrates and inhibitors of P-glycoprotein, nitrates, sulphonylureas, loop diuretics, potassium sparing diuretics, thiazide diuretics, substrates and inhibitors of tubular organic cation transport, and QTc-prolonging drugs. Differences in clearance between patients on these medications (at any occasion in the study) and those off medications varied between -16% and +3%. The mean clearances of dofetilide were 16% and 15% lower in patients on thiazide diuretics and inhibitors of tubular organic cation transport, respectively.
- [Geriatric Use]
- Of the total number of patients in clinical studies of dofetilide, 46% were 65 to 89 years old. No overall differences in safety, effect on QTc, or effectiveness were observed between elderly and younger patients. Because elderly patients are more likely to have decreased renal function with a reduced creatinine clearance, care must be taken in dose selection (see DOSAGE AND ADMINISTRATION).
- CONTRAINDICATIONS
- Dofetilide is contraindicated in patients with congenital or acquired long QT syndromes. Dofetilide should not be used in patients with a baseline QT interval or QTc >440 msec (500 msec in patients with ventricular conduction abnormalities). Dofetilide is also contraindicated in patients with severe renal impairment (calculated creatinine clearance <20 mL/min).
- The concomitant use of verapamil or the cation transport system inhibitors cimetidine, trimethoprim (alone or in combination with sulfamethoxazole), or ketoconazole with dofetilide is contraindicated (see WARNINGS and PRECAUTIONS, Drug-Drug Interactions), as each of these drugs cause a substantial increase in dofetilide plasma concentrations. In addition, other known inhibitors of the renal cation transport system such as prochlorperazine, dolutegravir and megestrol should not be used in patients on dofetilide.
- The concomitant use of hydrochlorothiazide (alone or in combinations such as with triamterene) with dofetilide is contraindicated (see PRECAUTIONS, Drug-Drug Interactions) because this has been shown to significantly increase dofetilide plasma concentrations and QT interval prolongation.
- Dofetilide is also contraindicated in patients with a known hypersensitivity to the drug.
- OVERDOSAGE
- There is no known antidote to dofetilide; treatment of overdose should therefore be symptomatic and supportive. The most prominent manifestation of overdosage is likely to be excessive prolongation of the QT interval.
- In cases of overdose, cardiac monitoring should be initiated. Charcoal slurry may be given soon after overdosing but has been useful only when given within 15 minutes of dofetilide administration. Treatment of Torsade de Pointes or overdose may include administration of isoproterenol infusion, with or without cardiac pacing. Administration of intravenous magnesium sulfate may be effective in the management of Torsade de Pointes. Close medical monitoring and supervision should continue until the QT interval returns to normal levels.
- DOSAGE AND ADMINISTRATION
- The dose of dofetilide capsules must be individualized according to calculated creatinine clearance and QTc. (QT interval should be used if the heart rate is <60 beats per minute. There are no data on use of dofetilide capsules when the heart rate is <50 beats per minute.) The usual recommended dose of dofetilide capsules is 500 mcg BID, as modified by the dosing algorithm described below. For consideration of a lower dose, see Special Considerations below.
- [Initiation of Dofetilide Capsules Therapy]
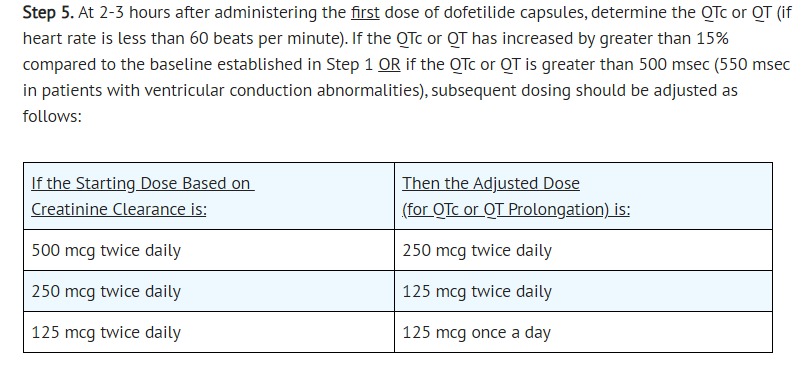
- CLINICAL PHARMACOLOGY
- Electrophysiology
- Dofetilide increases the monophasic action potential duration in a predictable, concentration-dependent manner, primarily due to delayed repolarization. This effect, and the related increase in effective refractory period, is observed in the atria and ventricles in both resting and paced electrophysiology studies. The increase in QT interval observed on the surface ECG is a result of prolongation of both effective and functional refractory periods in the His-Purkinje system and the ventricles.
- [Renal Impairment]
- In volunteers with varying degrees of renal impairment and patients with arrhythmias, the clearance of dofetilide decreases with decreasing creatinine clearance. As a result, and as seen in clinical studies, the half-life of dofetilide is longer in patients with lower creatinine clearances. Because increase in QT interval and the risk of ventricular arrhythmias are directly related to plasma concentrations of dofetilide, dosage adjustment based on calculated creatinine clearance is critically important (see DOSAGE AND ADMINISTRATION) . Patients with severe renal impairment (creatinine clearance <20 mL/min) were not included in clinical or pharmacokinetic studies (see CONTRAINDICATIONS).
- [Dose-Response and Concentration Response for Increase in QT Interval]
- MEDICATION GUIDE
- Who should not take dofetilide capsules?
- Do not take dofetilide capsules if you:
- have an irregular heartbeat called long QT syndrome
- have kidney problems or are on kidney dialysis
- take any of these medicines:
- cimetidine (TAGAMET, TAGAMET HB)*
- verapamil (CALAN, CALAN SR, COVERA-HS, ISOPTIN, ISOPTIN SR, VERELAN, VERELAN PM, TARKA)*
- ketoconazole (NIZORAL, XOLEGEL, EXTINA)*
- trimethoprim alone (PROLOPRIM, TRIMPEX)* or the combination of trimethoprim and sulfamethoxazole (BACTRIM, SEPTRA SULFATRIM)*
- prochlorperazine (COMPAZINE, COMPO)*
- megestrol (MEGACE)*
- dolutegravir (TIVICAY)*
- hydrochlorothiazide alone or in combination with other medicines (such as ESIDRIX, EZIDE, HYDRODIURIL, HYDRO-PAR, MICROZIDE, or ORETIC)*
- CLINICAL STUDIES
- Chronic Atrial Fibrillation and/or Atrial Flutter
- Two randomized, parallel, double-blind, placebo-controlled, dose-response trials evaluated the ability of dofetilide 1) to convert patients with atrial fibrillation or atrial flutter (AF/AFl) of more than 1 week duration to normal sinus rhythm (NSR) and 2) to maintain NSR (delay time to recurrence of AF/AFl) after drug-induced or electrical cardioversion. A total of 996 patients with a one week to two year history of atrial fibrillation/atrial flutter were enrolled. Both studies randomized patients to placebo or to doses of dofetilide 125 mcg, 250 mcg, 500 mcg, or in one study a comparator drug, given twice a day (these doses were lowered based on calculated creatinine clearance and, in one of the studies, for QT interval or QTc). All patients were started on therapy in a hospital where their ECG was monitored (see DOSAGE AND ADMINISTRATION).
- Patients were excluded from participation if they had had syncope within the past 6 months, AV block greater than first degree, MI or unstable angina within 1 month, cardiac surgery within 2 months, history of QT interval prolongation or polymorphic ventricular tachycardia associated with use of antiarrhythmic drugs, QT interval or QTc >440 msec, serum creatinine >2.5 mg/mL, significant diseases of other organ systems; used cimetidine; or used drugs known to prolong the QT interval.
- Both studies enrolled mostly Caucasians (over 90%), males (over 70%), and patients ≥65 years of age (over 50%). Most (>90%) were NYHA Functional Class I or II. Approximately one-half had structural heart disease (including ischemic heart disease, cardiomyopathies, and valvular disease) and about one-half were hypertensive. A substantial proportion of patients were on concomitant therapy, including digoxin (over 60%), diuretics (over 20%), and ACE inhibitors (over 30%). About 90% were on anticoagulants.
- Acute conversion rates are shown in Table 1 for randomized doses (doses were adjusted for calculated creatinine clearance and, in Study 1, for QT interval or QTc). Of patients who converted pharmacologically, approximately 70% converted within 24-36 hours.
- In both studies, dofetilide resulted in a dose-related increase in the number of patients maintained in NSR at all time periods and delayed the time of recurrence of sustained AF. Data pooled from both studies show that there is a positive relationship between the probability of staying in NSR, dofetilide dose, and increase in QTc (see Figure 2 in CLINICAL PHARMACOLOGY, Dose-Response and Concentration Response for Increase in QT Interval).
- Analysis of pooled data for patients randomized to a dofetilide dose of 500 mcg twice daily showed that maintenance of NSR was similar in both males and females, in both patients aged <65 years and patients ≥65 years of age, and in both patients with atrial flutter as a primary diagnosis and those with a primary diagnosis of atrial fibrillation.
- During the period of in-hospital initiation of dosing, 23% of patients in Studies 1 and 2 had their dose adjusted downward on the basis of their calculated creatinine clearance, and 3% had their dose down-titrated due to increased QT interval or QTc. Increased QT interval or QTc led to discontinuation of therapy in 3% of patients.
- Safety in Patients with Structural Heart Disease: DIAMOND Studies (The Danish Investigations of Arrhythmia and Mortality on Dofetilide)
- In both DIAMOND studies, patients were randomized to 500 mcg BID of dofetilide, but this was reduced to 250 mcg BID if calculated creatinine clearance was 40–60 mL/min, if patients had AF, or if QT interval prolongation (>550 msec or >20% increase from baseline) occurred after dosing. Dose reductions for reduced calculated creatinine clearance occurred in 47% and 45% of DIAMOND CHF and MI patients, respectively. Dose reductions for increased QT interval or QTc occurred in 5% and 7% of DIAMOND CHF and MI patients, respectively. Increased QT interval or QTc (>550 msec or >20% increase from baseline) resulted in discontinuation of 1.8% of patients in DIAMOND CHF and 2.5% of patients in DIAMOND MI.
- In the DIAMOND studies, all patients were hospitalized for at least 3 days after treatment was initiated and monitored by telemetry. Patients with QTc greater than 460 msec, second or third degree AV block (unless with pacemaker), resting heart rate <50 bpm, or prior history of polymorphic ventricular tachycardia were excluded.
- Of the 506 patients in the DIAMOND studies who had atrial fibrillation or flutter at baseline, 12% of patients in the dofetilide group and 2% of patients in the placebo group had converted to normal sinus rhythm after one month. In those patients converted to normal sinus rhythm, 79% of the dofetilide group and 42% of the placebo group remained in normal sinus rhythm for one year.
- In the DIAMOND studies, although Torsade de Pointes occurred more frequently in the dofetilide-treated patients (see ADVERSE REACTIONS), dofetilide, given with an initial 3-day hospitalization and with dose modified for reduced creatinine clearance and increased QT interval, was not associated with an excess risk of mortality in these populations with structural heart disease in the individual studies or in an analysis of the combined studies. The presence of atrial fibrillation did not affect outcome.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
327
23765
Other ADRs
15112
38366475
Odds Ratio = 34.934
Drug Property Information
ATC Code(s):
- C01BD04 - dofetilide
- C01BD - "Antiarrhythmics, class III"
- C01B - "ANTIARRHYTHMICS, CLASS I AND III"
- C01 - CARDIAC THERAPY
- C - CARDIOVASCULAR SYSTEM
Active Ingredient:DOFETILIDE
Active Ingredient UNII:R4Z9X1N2ND
Drugbank ID:DB00204
PubChem Compound:71329
CTD ID:C063533
PharmGKB:PA449389
CAS Number:115256-11-6
Dosage Form(s):capsule
Route(s) Of Administrator:oral
Daily Dose:
Chemical Structure: 

SMILE Code:
CN(CCOC1=CC=C(NS(C)(=O)=O)C=C1)CCC1=CC=C(NS(C)(=O)=O)C=C1
CN(CCOC1=CC=C(NS(C)(=O)=O)C=C1)CCC1=CC=C(NS(C)(=O)=O)C=C1
Reference
1: Safety of Inpatient Dofetilide Initiation per Cardiology Services: A Retrospective Review.
[Cicirale Carrie,Jackson Jennifer,Gothard David]J Pharm Pract,2021 Mar 19;8971900211000212. PMID: 33736527
2: QT Dispersion and Drug-Induced Torsade de Pointes.
[Friedman Ari,Miles Jeremy,Liebelt Jared,Christia Panagiota,Engstrom Krysthel,Thachil Rosy,Grushko Michael,Faillace Robert T]Cureus,2021 Jan 25;13(1):e12895. PMID: 33643739
3: A powerful test for the maximum treatment effect in thorough QT/QTc studies.
[Deng Yuhao,Chen Fangyi,Li Yang,Qian Kaihuan,Wang Rui,Zhou Xiao-Hua]Stat Med,2021 Apr 15;40(8):1947-1959. PMID: 33463746
4: Identification of Drug-Induced Multichannel Block and Proarrhythmic Risk in Humans Using Continuous T Vector Velocity Effect Profiles Derived From Surface Electrocardiograms.
[Bystricky Werner,Maier Christoph,Gintant Gary,Bergau Dennis,Carter David]Front Physiol,2020 Sep 18;11:567383. PMID: 33071822
5: Evaluation of a Fully Automatic Measurement of Short-Term Variability of Repolarization on Intracardiac Electrograms in the Chronic Atrioventricular Block Dog.
[Smoczyńska Agnieszka,Sprenkeler David J,Aranda Alfonso,Beekman Jet D M,Bossu Alexandre,Dunnink Albert,Wijers Sofieke C,Stegemann Berthold,Meine Mathias,Vos Marc A]Front Physiol,2020 Aug 21;11:1005. PMID: 32973549
6: Clinical Outcomes and Characteristics With Dofetilide in Atrial Fibrillation Patients Considered for Implantable Cardioverter-Defibrillator.
[Koene Ryan J,Menon Vivek,Cantillon Daniel J,Dresing Thomas J,Martin David O,Kanj Mohamed,Saliba Walid I,Tarakji Khaldoun G,Baranowski Bryan,Hussein Ayman A,Tchou Patrick J,Bhargava Mandeep,Callahan Thomas D,Rickard John W,Niebauer Mark J,Chung Mina K,Varma Niraj,Wilkoff Bruce L,Lindsay Bruce D,Wazni Oussama M]Circ Arrhythm Electrophysiol,2020 Jul;13(7):e008168. PMID: 32538135
7: Assessment of Sotalol and Dofetilide Dosing at a Large Academic Medical Center.
[Ting Clara,Malloy Rhynn,Knowles Danielle]J Cardiovasc Pharmacol Ther,2020 Sep;25(5):438-443. PMID: 32347108
8: A Computational Pipeline to Predict Cardiotoxicity: From the Atom to the Rhythm.
[Yang Pei-Chi,DeMarco Kevin R,Aghasafari Parya,Jeng Mao-Tsuen,Dawson John R D,Bekker Slava,Noskov Sergei Y,Yarov-Yarovoy Vladimir,Vorobyov Igor,Clancy Colleen E]Circ Res,2020 Apr 10;126(8):947-964. PMID: 32091972
9: Dofetilide-Induced Microvolt T-Wave Alternans.
[Marcantoni Ilaria,Laratta Rosita,Mascia Guido,Ricciardi Leonardo,Sbrollini Agnese,Nasim Amnah,Morettini Micaela,Burattini Laura]Annu Int Conf IEEE Eng Med Biol Soc,2019 Jul;2019:95-98. PMID: 31945853
10: {'#text': 'Three-Dimensional Heart Model-Based Screening of Proarrhythmic Potential by Simulation of Action Potential and Electrocardiograms.', 'i': 'in silico'}
[Hwang Minki,Han Seunghoon,Park Min Cheol,Leem Chae Hun,Shim Eun Bo,Yim Dong-Seok]Front Physiol,2019 Sep 4;10:1139. PMID: 31551815
11: Cardiotoxicity screening of illicit drugs and new psychoactive substances (NPS) in human iPSC-derived cardiomyocytes using microelectrode array (MEA) recordings.
[Zwartsen Anne,de Korte Tessa,Nacken Peter,de Lange Dylan W,Westerink Remco H S,Hondebrink Laura]J Mol Cell Cardiol,2019 Nov;136:102-112. PMID: 31526813
12: Pro-Arrhythmic Ventricular Remodeling Is Associated With Increased Respiratory and Low-Frequency Oscillations of Monophasic Action Potential Duration in the Chronic Atrioventricular Block Dog Model.
[Sprenkeler David Jaap,Beekman Jet D M,Bossu Alexandre,Dunnink Albert,Vos Marc A]Front Physiol,2019 Aug 23;10:1095. PMID: 31507455
13: Classification of drug-induced hERG potassium-channel block from electrocardiographic T-wave features using artificial neural networks.
[Morettini Micaela,Peroni Chiara,Sbrollini Agnese,Marcantoni Ilaria,Burattini Laura]Ann Noninvasive Electrocardiol,2019 Nov;24(6):e12679. PMID: 31347753
14: Clinical Trial in a Dish: Personalized Stem Cell-Derived Cardiomyocyte Assay Compared With Clinical Trial Results for Two QT-Prolonging Drugs.
[Blinova Ksenia,Schocken Derek,Patel Dakshesh,Daluwatte Chathuri,Vicente Jose,Wu Joseph C,Strauss David G]Clin Transl Sci,2019 Nov;12(6):687-697. PMID: 31328865
15: T vector velocity: A new ECG biomarker for identifying drug effects on cardiac ventricular repolarization.
[Bystricky Werner,Maier Christoph,Gintant Gary,Bergau Dennis,Kamradt Kent,Welsh Patrick,Carter David]PLoS One,2019 Jul 8;14(7):e0204712. PMID: 31283756
16: {'#text': 'The K2 Channel Inhibitor AP14145, But Not Dofetilide or Ondansetron, Provides Functional Atrial Selectivity in Guinea Pig Hearts.', 'sub': 'Ca'}
[Kirchhoff Jeppe Egedal,Skarsfeldt Mark Alexander,Muthukumarasamy Kalai Mangai,Simó-Vicens Rafel,Bomholtz Sofia Hammami,Abildgaard Lea,Jespersen Thomas,Sørensen Ulrik S,Grunnet Morten,Bentzen Bo Hjorth,Diness Jonas Goldin]Front Pharmacol,2019 Jun 19;10:668. PMID: 31275147
17: Exposure-response analysis of drug-induced QT interval prolongation in telemetered monkeys for translational prediction to human.
[Komatsu Ryuichi,Mizuno Hiroshi,Ishizaka Tomomichi,Ito Akihito,Jikuzono Tatsuya,Kakoi Tadashi,Bando Masahiro,Koga Tadashi,Handa Jun,Takahashi Yukio,Kanno Akihiro,Ozaki Harushige,Chiba Katsuyoshi,Japan activity for Improvement of Cardiovascular Evaluation by Telemetry system (J-ICET)]J Pharmacol Toxicol Methods,Sep-Oct 2019;99:106606. PMID: 31255745
18: Pharmacist-Managed Inpatient Dofetilide Initiation Program: Description and Adherence Rate Post-Root Cause Analysis.
[Ko Elena You Jung,Carpenter Charles M,Gagnon David J,Andrle Anne M]J Pharm Pract,2020 Dec;33(6):784-789. PMID: 30935279
19: Safety of rapid switching from amiodarone to dofetilide in atrial fibrillation patients with an implantable cardioverter-defibrillator.
[Sharma Sharan Prakash,Turagam Mohit,Atkins Donita,Bommana Sudha,Jeffrey Courtney,Newton Diana,Nydegger Cheri,Carroll Heidi,Gopinathannair Rakesh,Natale Andrea,Lakkireddy Dhanunjaya]Heart Rhythm,2019 Jul;16(7):990-995. PMID: 30710741
20: Stroke or side effect? dofetilide associated facial paralysis after direct current cardioversion for atrial fibrillation.
[Zhang Lane,Steckman David]BMJ Case Rep,2019 Jan 29;12(1):e227705. PMID: 30700466
21: Potentially modifiable factors of dofetilide-associated risk of torsades de pointes among hospitalized patients with atrial fibrillation.
[Naksuk Niyada,Sugrue Alan M,Padmanabhan Deepak,Kella Danesh,DeSimone Christopher V,Kapa Suraj,Asirvatham Samuel J,Lee Hon-Chi,Ackerman Michael J,Noseworthy Peter A]J Interv Card Electrophysiol,2019 Mar;54(2):189-196. PMID: 30353374
22: An Augmented Negative Force-Frequency Relationship and Slowed Mechanical Restitution Are Associated With Increased Susceptibility to Drug-Induced Torsade de Pointes Arrhythmias in the Chronic Atrioventricular Block Dog.
[Sprenkeler David J,Bossu Alexandre,Beekman Jet D M,Schoenmakers Marieke,Vos Marc A]Front Physiol,2018 Aug 8;9:1086. PMID: 30135660
23: Dofetilide-Associated QT Prolongation: Total Body Weight Versus Adjusted or Ideal Body Weight for Dosing.
[Wang Stephen Y,Welch Terrence D,Sangha Rajbir S,Maloney Robert W,Cui Zhu,Kaplan Aaron V]J Cardiovasc Pharmacol,2018 Sep;72(3):161-165. PMID: 29985283
24: Whole-exome sequencing reveals microsatellite DNA markers for response to dofetilide initiation in patients with persistent atrial fibrillation: A pilot study.
[Kinney Nick,Larsen Timothy R,Kim David M,Varghese Robin T,Poelzing Steven,Garner Harold R,AlMahameed Soufian T]Clin Cardiol,2018 Jun;41(6):849-854. PMID: 29671888
25: Dofetilide dose reductions and discontinuations in women compared with men.
[Pokorney Sean D,Yen Debbie C,Campbell Kristen B,Allen LaPointe Nancy M,Sheng Shubin,Thomas Laine,Bahnson Tristram D,Daubert James P,Picini Jonathan P,Jackson Kevin P,Thomas Kevin L,Al-Khatib Sana M]Heart Rhythm,2018 Apr;15(4):478-484. PMID: 29605013
26: Predicting the cardiac toxicity of drugs using a novel multiscale exposure-response simulator.
[Sahli Costabal Francisco,Yao Jiang,Kuhl Ellen]Comput Methods Biomech Biomed Engin,2018 Feb;21(3):232-246. PMID: 29493299
27: Safety and Efficacy of Inpatient Initiation of Dofetilide versus Sotalol for atrial fibrillation.
[Yarlagadda Bharath,Vuddanda Venkat,Dar Tawseef,Jazayeri Mohammad-Ali,Parikh Valay,Turagam Mohit K,Lavu Madhav,Avula Sindhu Reddy,Atkins Donita,Bommana Sudharani,Gopinathannair Rakesh,Yeruva Madhu Reddy,Lakkireddy Dhanunjaya]J Atr Fibrillation,2017 Dec 31;10(4):1805. PMID: 29487686
28: Dofetilide for suppression of atrial fibrillation in hypertrophic cardiomyopathy: A case series and literature review.
[Moore JoEllyn C,Trager Lena,Anzia Lucille E,Saliba Walid,Bassiouny Mohamed,Bhargava Mandeep,Chung Mina,Desai Milind,Garberich Ross,Lever Harry,Lindsay Bruce D,Sengupta Jay,Tchou Patrick,Wazni Oussama,Wilkoff Bruce L]Pacing Clin Electrophysiol,2018 Apr;41(4):396-401. PMID: 29450893
29: An evaluation of multiple algorithms for the measurement of the heart rate corrected JTpeak interval.
[Couderc Jean-Philippe,Ma Shiyang,Page Alex,Besaw Connor,Xia Jean,Chiu W Brian,de Bie Johan,Vicente Jose,Vaglio Martino,Badilini Fabio,Babaeizadeh Saeed,Chien Cheng-Hao Simon,Baumert Mathias]J Electrocardiol,Nov-Dec 2017;50(6):769-775. PMID: 29021091
30: The algorithmic performance of J-Tpeak for drug safety clinical trial.
[Chien Simon C,Gregg Richard E]J Electrocardiol,Nov-Dec 2017;50(6):762-768. PMID: 28942951
31: Electrocardiographic biomarkers to confirm drug's electrophysiological effects used for proarrhythmic risk prediction under CiPA.
[Vicente Jose,Hosseini Meisam,Johannesen Lars,Strauss David G]J Electrocardiol,Nov-Dec 2017;50(6):808-813. PMID: 28928044
32: Drug-Induced QT Prolongation and Torsades de Pointes: An All-Exclusive Relationship or Time for an Amicable Separation?
[Hondeghem Luc M]Drug Saf,2018 Jan;41(1):11-17. PMID: 28853038
33: Characterization of loperamide-mediated block of hERG channels at physiological temperature and its proarrhythmia propensity.
[Sheng Jiansong,Tran Phu N,Li Zhihua,Dutta Sara,Chang Kelly,Colatsky Thomas,Wu Wendy W]J Pharmacol Toxicol Methods,Nov-Dec 2017;88(Pt 2):109-122. PMID: 28830713
34: Dofetilide-induced torsade de pointes in high-grade atrioventricular node dysfunction.
[Alsaad Ali A,Silvers Scott M,Kusumoto Fred,Blackshear Joseph L]Postgrad Med J,2017 Oct;93(1104):635-636. PMID: 28663351
35: Deleterious acute and chronic effects of bradycardic right ventricular apex pacing: consequences for arrhythmic outcome.
[Stams T R G,Dunnink A,van Everdingen W M,Beekman H D M,van der Nagel R,Kok B,Bierhuizen M F A,Cramer M J,Meine M,Vos M A]Basic Res Cardiol,2017 Jul;112(4):46. PMID: 28624975
36: The anaesthetized rabbit with acute atrioventricular block provides a new model for detecting drug-induced Torsade de Pointes.
[Hagiwara Mihoko,Shibuta Seiji,Takada Kazuhiro,Kambayashi Ryuichi,Nakajo Misako,Aimoto Megumi,Nagasawa Yoshinobu,Takahara Akira]Br J Pharmacol,2017 Aug;174(15):2591-2605. PMID: 28547743
37: Torsade de pointes arrhythmias arise at the site of maximal heterogeneity of repolarization in the chronic complete atrioventricular block dog.
[Dunnink Albert,Stams Thom R G,Bossu Alexandre,Meijborg Veronique M F,Beekman Jet D M,Wijers Sofieke C,De Bakker Jacques M T,Vos Marc A]Europace,2017 May 1;19(5):858-865. PMID: 28525920
38: Synergistic Effect of Dofetilide and Mexiletine on Prevention of Atrial Fibrillation.
[Liu Guizhi,Xue Xiaolin,Gao Chuanyu,Huang Jiaqi,Qi Datun,Zhang Yanzhou,Dong Jian-Zeng,Ma Chang-Sheng,Yan Gan-Xin]J Am Heart Assoc,2017 May 18;6(5):e005482. PMID: 28522677
39: A multiscale computational modelling approach predicts mechanisms of female sex risk in the setting of arousal-induced arrhythmias.
[Yang Pei-Chi,Perissinotti Laura L,López-Redondo Fernando,Wang Yibo,DeMarco Kevin R,Jeng Mao-Tsuen,Vorobyov Igor,Harvey Robert D,Kurokawa Junko,Noskov Sergei Y,Clancy Colleen E]J Physiol,2017 Jul 15;595(14):4695-4723. PMID: 28516454
40: Proarrhythmic risk assessment using conventional and new in vitro assays.
[Goineau Sonia,Castagné Vincent]Regul Toxicol Pharmacol,2017 Aug;88:1-11. PMID: 28506844
41: Practice variation in the re-initiation of dofetilide: An observational study.
[Turagam Mohit K,Afzal Muhammad R,Reddy Madhu,Pillarisetti Jayasree,Lavu Madhav,Atkins Donita,Jeffrey Courtney,Christensen Katie,Pimentel Rhea,Dendi Raghuveer,Vacek James,Hurwitz Jodi,Di Biase Luigi,Natale Andrea,Lakkireddy Dhanunjaya]Int J Cardiol,2017 Jun 1;236:221-225. PMID: 28233630
42: Pharmacologic Conversion during Dofetilide Treatment for Persistent Atrial Fibrillation.
[Steinberg Jonathan S,Shah Yash,Szepietowska Barbara]Pacing Clin Electrophysiol,2017 Jun;40(6):667-671. PMID: 28220940
43: Common Genetic Variant Risk Score Is Associated With Drug-Induced QT Prolongation and Torsade de Pointes Risk: A Pilot Study.
[Strauss David G,Vicente Jose,Johannesen Lars,Blinova Ksenia,Mason Jay W,Weeke Peter,Behr Elijah R,Roden Dan M,Woosley Ray,Kosova Gulum,Rosenberg Michael A,Newton-Cheh Christopher]Circulation,2017 Apr 4;135(14):1300-1310. PMID: 28213480
44: Electrophysiological measurements that can explain and guide temporary accelerated pacing to avert (re)occurrence of torsade de pointes arrhythmias in the canine chronic atrioventricular block model.
[Wijers Sofieke C,Bossu Alexandre,Dunnink Albert,Beekman Jet D M,Varkevisser Rosanne,Aranda Hernández Alfonso,Meine Mathias,Vos Marc A]Heart Rhythm,2017 May;14(5):749-756. PMID: 28213055
45: Application of a systems pharmacology model for translational prediction of hERG-mediated QTc prolongation.
[Gotta Verena,Yu Zhiyi,Cools Frank,van Ammel Karel,Gallacher David J,Visser Sandra A G,Sannajust Frederick,Morissette Pierre,Danhof Meindert,van der Graaf Piet H]Pharmacol Res Perspect,2016 Nov 17;4(6):e00270. PMID: 28097003
46: Electrocardiographic Biomarkers for Detection of Drug-Induced Late Sodium Current Block.
[Vicente Jose,Johannesen Lars,Hosseini Meisam,Mason Jay W,Sager Philip T,Pueyo Esther,Strauss David G]PLoS One,2016 Dec 30;11(12):e0163619. PMID: 28036334
47: Glass Half Empty?
[Kowey Peter R,Shah Ashish M]JACC Clin Electrophysiol,2016 Dec;2(7):782-783. PMID: 29759760
48: Discontinuation of Dofetilide From QT Prolongation and Ventricular Tachycardia in the Real World.
[Anand Vidhu,Vakil Kairav,Tholakanahalli Venkatakrishna,Li Jian-Ming,McFalls Edward,Adabag Selcuk]JACC Clin Electrophysiol,2016 Dec;2(7):777-781. PMID: 29759759
49: Significance of integrated in silico transmural ventricular wedge preparation models of human non-failing and failing hearts for safety evaluation of drug candidates.
[Kubo Taeko,Ashihara Takashi,Tsubouchi Tadashi,Horie Minoru]J Pharmacol Toxicol Methods,Jan-Feb 2017;83:30-41. PMID: 27546811
50: Role of the pH in state-dependent blockade of hERG currents.
[Wang Yibo,Guo Jiqing,Perissinotti Laura L,Lees-Miller James,Teng Guoqi,Durdagi Serdar,Duff Henry J,Noskov Sergei Yu]Sci Rep,2016 Oct 12;6:32536. PMID: 27731415
51: {'#text': 'Antiarrhythmic effect of the Ca-activated K (SK) channel inhibitor ICA combined with either amiodarone or dofetilide in an isolated heart model of atrial fibrillation.', 'sup': ['2+', '+']}
[Kirchhoff Jeppe Egedal,Diness Jonas Goldin,Abildgaard Lea,Sheykhzade Majid,Grunnet Morten,Jespersen Thomas]Pflugers Arch,2016 Nov;468(11-12):1853-1863. PMID: 27722784
52: Dofetilide in Overdose: A Case Series from Poison Center Data.
[Hieger M A,Maskell K F,Moss M J,Powell S W,Cumpston K L]Cardiovasc Toxicol,2017 Jul;17(3):368-371. PMID: 27565970
53: Use of dofetilide in adult patients with atrial arrhythmias and congenital heart disease: A PACES collaborative study.
[El-Assaad Iqbal,Al-Kindi Sadeer G,Abraham JoEllyn,Sanatani Shubhayan,Bradley David J,Halsey Colby,Law Ian H,Balaji Seshadri,Shetty Ira,Aziz Peter F]Heart Rhythm,2016 Oct;13(10):2034-9. PMID: 27435587
54: Pharmacokinetic-pharmacodynamic modelling of drug-induced QTc interval prolongation in man: prediction from in vitro human ether-à-go-go-related gene binding and functional inhibition assays and conscious dog studies.
[Dubois V F S,Casarotto E,Danhof M,Della Pasqua O]Br J Pharmacol,2016 Oct;173(19):2819-32. PMID: 27427789
55: The QT Scale: A Weight Scale Measuring the QTc Interval.
[Couderc Jean-Philippe,Beshaw Connor,Niu Xiaodan,Serrano-Finetti Ernesto,Casas Oscar,Pallas-Areny Ramon,Rosero Spencer,Zareba Wojciech]Ann Noninvasive Electrocardiol,2017 Jan;22(1):e12378. PMID: 27422673
56: Use of FDSS/μCell imaging platform for preclinical cardiac electrophysiology safety screening of compounds in human induced pluripotent stem cell-derived cardiomyocytes.
[Zeng Haoyu,Roman Maria I,Lis Edward,Lagrutta Armando,Sannajust Frederick]J Pharmacol Toxicol Methods,Sep-Oct 2016;81:217-22. PMID: 27222351
57: Beat-to-Beat Variability in Preload Unmasks Latent Risk of Torsade de Pointes in Anesthetized Chronic Atrioventricular Block Dogs.
[Stams Thom Rg,Oosterhoff Peter,Heijdel Atty,Dunnink Albert,Beekman Jet Dm,van der Nagel Roel,van Rijen Harold Vm,van der Heyden Marcel Ag,Vos Marc A]Circ J,2016 May 25;80(6):1336-45. PMID: 27151565
58: Specific prediction of clinical QT prolongation by kinetic image cytometry in human stem cell derived cardiomyocytes.
[Pfeiffer Emily R,Vega Raquel,McDonough Patrick M,Price Jeffrey H,Whittaker Ross]J Pharmacol Toxicol Methods,Sep-Oct 2016;81:263-73. PMID: 27095424
59: A novel transgenic rabbit model with reduced repolarization reserve: long QT syndrome caused by a dominant-negative mutation of the KCNE1 gene.
[Major Péter,Baczkó István,Hiripi László,Odening Katja E,Juhász Viktor,Kohajda Zsófia,Horváth András,Seprényi György,Kovács Mária,Virág László,Jost Norbert,Prorok János,Ördög Balázs,Doleschall Zoltán,Nattel Stanley,Varró András,Bősze Zsuzsanna]Br J Pharmacol,2016 Jun;173(12):2046-61. PMID: 27076034
60: New in vitro model for proarrhythmia safety screening: IKs inhibition potentiates the QTc prolonging effect of IKr inhibitors in isolated guinea pig hearts.
[Kui Péter,Orosz Szabolcs,Takács Hedvig,Sarusi Annamária,Csík Norbert,Rárosi Ferenc,Csekő Csongor,Varró András,Papp Julius Gy,Forster Tamás,Farkas Attila S,Farkas András]J Pharmacol Toxicol Methods,Jul-Aug 2016;80:26-34. PMID: 27063345
61: Comparison of QT Interval Readings in Normal Sinus Rhythm Between a Smartphone Heart Monitor and a 12-Lead ECG for Healthy Volunteers and Inpatients Receiving Sotalol or Dofetilide.
[Garabelli Paul,Stavrakis Stavros,Albert Michael,Koomson Edward,Parwani Purvi,Chohan Jawad,Smith Landgrave,Albert David,Xie Rongsheng,Xie Qiying,Reynolds Dwight,Po Sunny]J Cardiovasc Electrophysiol,2016 Jul;27(7):827-32. PMID: 27027653
62: Quantitative Profiling of the Effects of Vanoxerine on Human Cardiac Ion Channels and its Application to Cardiac Risk.
[Obejero-Paz Carlos A,Bruening-Wright Andrew,Kramer James,Hawryluk Peter,Tatalovic Milos,Dittrich Howard C,Brown Arthur M]Sci Rep,2015 Nov 30;5:17623. PMID: 26616666
63: Electrocardiographic Predictors of Torsadogenic Risk During Dofetilide or Sotalol Initiation: Utility of a Novel T Wave Analysis Program.
[Sugrue Alan,Kremen Vaclav,Qiang Bo,Sheldon Seth H,DeSimone Christopher V,Sapir Yehu,Striemer Bryan L,Brady Peter,Asirvatham Samuel J,Ackerman Michael J,Friedman Paul,Noseworthy Peter A]Cardiovasc Drugs Ther,2015;29(5):433-41. PMID: 26411977
64: Sex differences in drug-induced changes in ventricular repolarization.
[Vicente Jose,Johannesen Lars,Mason Jay W,Pueyo Esther,Stockbridge Norman,Strauss David G]J Electrocardiol,Nov-Dec 2015;48(6):1081-7. PMID: 26324176
65: Late sodium current block for drug-induced long QT syndrome: Results from a prospective clinical trial.
[Johannesen L,Vicente J,Mason J W,Erato C,Sanabria C,Waite-Labott K,Hong M,Lin J,Guo P,Mutlib A,Wang J,Crumb W J,Blinova K,Chan D,Stohlman J,Florian J,Ugander M,Stockbridge N,Strauss D G]Clin Pharmacol Ther,2016 Feb;99(2):214-23. PMID: 26259627
66: Implications of the IQ-CSRC Prospective Study: Time to Revise ICH E14.
[Darpo Borje,Garnett Christine,Keirns James,Stockbridge Norman]Drug Saf,2015 Sep;38(9):773-80. PMID: 26162419
67: Inter-study variability of preclinical in vivo safety studies and translational exposure-QTc relationships--a PKPD meta-analysis.
[Gotta V,Cools F,van Ammel K,Gallacher D J,Visser S A G,Sannajust F,Morissette P,Danhof M,van der Graaf P H]Br J Pharmacol,2015 Sep;172(17):4364-79. PMID: 26076100
68: Safety of oral dofetilide for rhythm control of atrial fibrillation and atrial flutter.
[Abraham JoEllyn M,Saliba Walid I,Vekstein Carolyn,Lawrence David,Bhargava Mandeep,Bassiouny Mohamed,Janiszewski David,Lindsay Bruce,Militello Michael,Nissen Steven E,Poe Stacy,Tanaka-Esposito Christine,Wolski Kathy,Wilkoff Bruce L]Circ Arrhythm Electrophysiol,2015 Aug;8(4):772-6. PMID: 26063741
69: Comprehensive T wave morphology assessment in a randomized clinical study of dofetilide, quinidine, ranolazine, and verapamil.
[Vicente Jose,Johannesen Lars,Mason Jay W,Crumb William J,Pueyo Esther,Stockbridge Norman,Strauss David G]J Am Heart Assoc,2015 Apr 13;4(4):e001615. PMID: 25870186
70: Dofetilide induced torsade de pointes: mechanism, risk factors and management strategies.
[Jaiswal Abhishek,Goldbarg Seth]Indian Heart J,Nov-Dec 2014;66(6):640-8. PMID: 25634399
71: Risk prediction for adverse events during initiation of sotalol and dofetilide for the treatment of atrial fibrillation.
[Agusala Kartik,Oesterle Adam,Kulkarni Chiraag,Caprio Timothy,Subacius Haris,Passman Rod]Pacing Clin Electrophysiol,2015 Apr;38(4):490-8. PMID: 25626340
72: Looking for virtuous promiscuity: electrocardiographic evidence of multichannel drug block.
[Haigney M C]Clin Pharmacol Ther,2014 Nov;96(5):534-6. PMID: 25336265
73: AV-block and conduction slowing prevail over TdP arrhythmias in the methoxamine-sensitized pro-arrhythmic rabbit model.
[Varkevisser Rosanne,Vos Marc A,Beekman Jet D,Tieland Ralph G,Van Der Heyden Marcel A]J Cardiovasc Electrophysiol,2015 Jan;26(1):82-9. PMID: 25154623
74: Induced KCNQ1 autoimmunity accelerates cardiac repolarization in rabbits: potential significance in arrhythmogenesis and antiarrhythmic therapy.
[Li Jin,Maguy Ange,Duverger James Elber,Vigneault Patrick,Comtois Philippe,Shi Yanfen,Tardif Jean-Claude,Thomas Dierk,Nattel Stanley]Heart Rhythm,2014 Nov;11(11):2092-100. PMID: 25087487
75: Differentiating drug-induced multichannel block on the electrocardiogram: randomized study of dofetilide, quinidine, ranolazine, and verapamil.
[Johannesen L,Vicente J,Mason J W,Sanabria C,Waite-Labott K,Hong M,Guo P,Lin J,Sørensen J S,Galeotti L,Florian J,Ugander M,Stockbridge N,Strauss D G]Clin Pharmacol Ther,2014 Nov;96(5):549-58. PMID: 25054430
76: The electromechanical window is no better than QT prolongation to assess risk of Torsade de Pointes in the complete atrioventricular block model in dogs.
[Stams T R G,Bourgonje V J A,Beekman H D M,Schoenmakers M,van der Nagel R,Oosterhoff P,van Opstal J M,Vos M A]Br J Pharmacol,2014 Feb;171(3):714-22. PMID: 24490860
77: QTc compared to JTc for monitoring drug-induced repolarization changes in the setting of ventricular pacing.
[Tsai Shane F,Houmsse Mahmoud,Dakhil Barrah,Augostini Ralph,Hummel John D,Kalbfleisch Steven J,Liu Zhengou,Love Charles,Rhodes Troy,Tyler Jaret,Weiss Raul,Hamam Ismail,Winner Marshall,Daoud Emile G]Heart Rhythm,2014 Mar;11(3):485-91. PMID: 24252288
78: Vernakalant is devoid of proarrhythmic effects in the complete AV block dog model.
[Varkevisser Rosanne,van der Heyden Marcel A G,Tieland Ralph G,Beekman Jet D M,Vos Marc A]Eur J Pharmacol,2013 Nov 15;720(1-3):49-54. PMID: 24211677
79: A fingerprint pair analysis of hERG inhibition data.
[Springer Clayton,Sokolnicki Katherine L]Chem Cent J,2013 Oct 21;7(1):167. PMID: 24144230
80: Clinical efficacy of dofetilide for the treatment of atrial tachyarrhythmias in adults with congenital heart disease.
[Banchs Javier E,Baquero Giselle A,Nickolaus Michelle J,Wolbrette Deborah L,Kelleman John J,Samii Soraya,Grando-Ting Jennifer,Penny-Peterson Erica,Davidson William R,Young Sallie K,Naccarelli Gerald V,Gonzalez Mario D]Congenit Heart Dis,May-Jun 2014;9(3):221-7. PMID: 23947935
81: Frequency of toxicity with chemical conversion of atrial fibrillation with dofetilide.
[Brumberg Genevieve,Gera Nitin,Pray Chris,Adelstein Evan,Barrington William,Bazaz Raveen,Mendenhall G Stuart,Nemec Jan,Voigt Andrew,Wang Norman C,Schwartzman David,Saba Samir,Jain Sandeep K]Am J Cardiol,2013 Aug 15;112(4):505-8. PMID: 23706388
82: Trends in reporting methadone-associated cardiac arrhythmia, 1997-2011: an analysis of registry data.
[Kao David,Bucher Bartelson Becki,Khatri Vaishali,Dart Richard,Mehler Philip S,Katz David,Krantz Mori J]Ann Intern Med,2013 May 21;158(10):735-40. PMID: 23689766
83: Translational pharmacokinetic-pharmacodynamic modeling of QTc effects in dog and human.
[Parkinson Joanna,Visser Sandra A G,Jarvis Philip,Pollard Chris,Valentin Jean-Pierre,Yates James W T,Ewart Lorna]J Pharmacol Toxicol Methods,Nov-Dec 2013;68(3):357-66. PMID: 23567074
84: Human embryonic stem cell derived cardiac myocytes detect hERG-mediated repolarization effects, but not Nav1.5 induced depolarization delay.
[Qu Yusheng,Gao Baoxi,Fang Mei,Vargas Hugo M]J Pharmacol Toxicol Methods,Jul-Aug 2013;68(1):74-81. PMID: 23518063
85: Combined Na(+)/Ca(2+) exchanger and L-type calcium channel block as a potential strategy to suppress arrhythmias and maintain ventricular function.
[Bourgonje Vincent J A,Vos Marc A,Ozdemir Semir,Doisne Nicolas,Acsai Karoly,Varro Andras,Sztojkov-Ivanov Anita,Zupko Istvan,Rauch Erik,Kattner Lars,Bito Virginie,Houtman Marien,van der Nagel Roel,Beekman Jet D,van Veen Toon A B,Sipido Karin R,Antoons Gudrun]Circ Arrhythm Electrophysiol,2013 Apr;6(2):371-9. PMID: 23515266
86: Wenxin Keli suppresses ventricular triggered arrhythmias via selective inhibition of late sodium current.
[Xue Xiaolin,Guo Donglin,Sun Hongmei,Wang Dan,Li Jiana,Liu Tengxian,Yang Lin,Shu Juan,Yan Gan-Xin]Pacing Clin Electrophysiol,2013 Jun;36(6):732-40. PMID: 23438075
87: A new biomarker--index of cardiac electrophysiological balance (iCEB)--plays an important role in drug-induced cardiac arrhythmias: beyond QT-prolongation and Torsades de Pointes (TdPs).
[Lu Hua Rong,Yan Gan-Xin,Gallacher David J]J Pharmacol Toxicol Methods,Sep-Oct 2013;68(2):250-259. PMID: 23337247
88: High-throughput screening of drug-binding dynamics to HERG improves early drug safety assessment.
[Di Veroli Giovanni Y,Davies Mark R,Zhang Henggui,Abi-Gerges Najah,Boyett Mark R]Am J Physiol Heart Circ Physiol,2013 Jan 1;304(1):H104-17. PMID: 23103500
89: LMI1195 PET imaging in evaluation of regional cardiac sympathetic denervation and its potential role in antiarrhythmic drug treatment.
[Yu Ming,Bozek Jody,Lamoy Melanie,Kagan Mikhail,Benites Pedro,Onthank David,Robinson Simon P]Eur J Nucl Med Mol Imaging,2012 Dec;39(12):1910-9. PMID: 22865199
90: Negative electro-mechanical windows are required for drug-induced Torsades de Pointes in the anesthetized guinea pig.
[Guns P-J,Johnson D M,Weltens E,Lissens J]J Pharmacol Toxicol Methods,2012 Sep;66(2):125-34. PMID: 22516473
91: The electro-mechanical window in anaesthetized guinea pigs: a new marker in screening for Torsade de Pointes risk.
[Guns P-J,Johnson D M,Van Op den Bosch J,Weltens E,Lissens J]Br J Pharmacol,2012 May;166(2):689-701. PMID: 22122450
92: Reduced ventricular proarrhythmic potential of the novel combined ion-channel blocker AZD1305 versus dofetilide in dogs with remodeled hearts.
[Johnson Daniel M,de Jong Monique M J,Crijns Harry J G M,Carlsson Leif G,Volders Paul G A]Circ Arrhythm Electrophysiol,2012 Feb;5(1):201-9. PMID: 22080293
93: Coronary artery disease potentiates response to dofetilide for rhythm control of atrial fibrillation.
[Manocha Pankaj,Bavikati Venkata,Langberg Jonathan,Lloyd Michael S]Pacing Clin Electrophysiol,2012 Feb;35(2):170-3. PMID: 22017595
94: Effects of K201 on repolarization and arrhythmogenesis in anesthetized chronic atrioventricular block dogs susceptible to dofetilide-induced torsade de pointes.
[Stams Thom R G,Oros Avram,der Nagel Roel van,Beekman Jet D M,Chamberlin Paul,Dittrich Howard C,Vos Marc A]Eur J Pharmacol,2011 Dec 15;672(1-3):126-34. PMID: 22001562
95: Ventricular remodelling is a prerequisite for the induction of dofetilide-induced torsade de pointes arrhythmias in the anaesthetized, complete atrio-ventricular-block dog.
[Dunnink Albert,van Opstal Jurren M,Oosterhoff Peter,Winckels Stephan K G,Beekman Jet D M,van der Nagel Roel,Cora Verduyn S,Vos Marc A]Europace,2012 Mar;14(3):431-6. PMID: 21946817
96: Comparison of the IKr blockers moxifloxacin, dofetilide and E-4031 in five screening models of pro-arrhythmia reveals lack of specificity of isolated cardiomyocytes.
[Nalos L,Varkevisser R,Jonsson M K B,Houtman M J C,Beekman J D,van der Nagel R,Thomsen M B,Duker G,Sartipy P,de Boer T P,Peschar M,Rook M B,van Veen T A B,van der Heyden M A G,Vos M A]Br J Pharmacol,2012 Jan;165(2):467-78. PMID: 21718297
97: Pharmacokinetic-pharmacodynamic modelling of the effect of Moxifloxacin on QTc prolongation in telemetered cynomolgus monkeys.
[Watson Kenny J,Gorczyca William P,Umland John,Zhang Ying,Chen Xian,Sun Sunny Z,Fermini Bernard,Holbrook Mark,Van Der Graaf Piet H]J Pharmacol Toxicol Methods,May-Jun 2011;63(3):304-13. PMID: 21419854
98: Regional genomic regulation of cardiac sodium-calcium exchanger by oestrogen.
[Chen Guojun,Yang Xiaoyan,Alber Sean,Shusterman Vladimir,Salama Guy]J Physiol,2011 Mar 1;589(Pt 5):1061-80. PMID: 21224239
99: BeKm-1, a peptide inhibitor of human ether-a-go-go-related gene potassium currents, prolongs QTc intervals in isolated rabbit heart.
[Qu Yusheng,Fang Mei,Gao Baoxi,Chui Ray W,Vargas Hugo M]J Pharmacol Exp Ther,2011 Apr;337(1):2-8. PMID: 21205913
100: Identification of human Ether-à-go-go related gene modulators by three screening platforms in an academic drug-discovery setting.
[Huang Xi-Ping,Mangano Thomas,Hufeisen Sandy,Setola Vincent,Roth Bryan L]Assay Drug Dev Technol,2010 Dec;8(6):727-42. PMID: 21158687
101: Sex and age related differences in drug induced QT prolongation by dofetilide under reduced repolarization reserve in simulated ventricular cells.
[Gonzalez Rodolfo,Gomis-Tena Julio,Corrias Alberto,Ferrero Jose Maria,Rodriguez Blanca,Saiz Javier]Annu Int Conf IEEE Eng Med Biol Soc,2010;2010:3245-8. PMID: 21096817
102: A KCR1 variant implicated in susceptibility to the long QT syndrome.
[Hayashi Kenshi,Fujino Noboru,Ino Hidekazu,Uchiyama Katsuharu,Sakata Kenji,Konno Tetsuo,Masuta Eiichi,Funada Akira,Sakamoto Yuichiro,Tsubokawa Toshinari,Hodatsu Akihiko,Yasuda Toshihiko,Kanaya Honin,Kim Min Young,Kupershmidt Sabina,Higashida Haruhiro,Yamagishi Masakazu]J Mol Cell Cardiol,2011 Jan;50(1):50-7. PMID: 20950623
103: Oscillations of cardiac wave length and proarrhythmia.
[Hondeghem L M,Dumotier B,Traebert M]Naunyn Schmiedebergs Arch Pharmacol,2010 Oct;382(4):367-76. PMID: 20803191
104: Robust anti-arrhythmic efficacy of verapamil and flunarizine against dofetilide-induced TdP arrhythmias is based upon a shared and a different mode of action.
[Oros A,Houtman M J,Neco P,Gomez A M,Rajamani S,Oosterhoff P,Attevelt N J,Beekman J D,van der Heyden M A G,Ver Donck L,Belardinelli L,Richard S,Antoons G,Vos M A,CONTICA investigators]Br J Pharmacol,2010 Sep;161(1):162-75. PMID: 20718748
105: Effects of sarcolemmal Ca(2+) entry, ryanodine function, and kinase inhibitors on a rabbit model of heart failure.
[Kijtawornrat Anusak,Ziolo Mark T,Nishijima Yoshinori,Roche Brian M,Hamlin Robert L]Int Heart J,2010 Jul;51(4):285-90. PMID: 20716847
106: Iatrogenic QT Abnormalities and Fatal Arrhythmias: Mechanisms and Clinical Significance.
[Cubeddu Luigi X]Curr Cardiol Rev,2009 Aug;5(3):166-76. PMID: 20676275
107: Worsening heart failure in the setting of dronedarone initiation.
[Coons James C,Plauger Kara M,Seybert Amy L,Sokos George G]Ann Pharmacother,2010 Sep;44(9):1496-500. PMID: 20628043
108: High-rate pacing reduces variability of repolarization and prevents repolarization-dependent arrhythmias in dogs with chronic AV block.
[Oosterhoff Peter,Thomsen Morten B,Maas Joep N,Atteveld Nico J M,Beekman Jet D M,VAN Rijen Harold V M,VAN DER Heyden Marcel A G,Vos Marc A]J Cardiovasc Electrophysiol,2010 Dec;21(12):1384-91. PMID: 20561108
109: Anesthesia and arrhythmogenesis in the chronic atrioventricular block dog model.
[Dunnink Albert,Sharif Shahnam,Oosterhoff Peter,Winckels Stephan,Montagne Denise,Beekman Jet,van der Nagel Roel,van der Heyden Marcel A G,Vos Marc A]J Cardiovasc Pharmacol,2010 Jun;55(6):601-8. PMID: 20555233
110: Beat-by-beat QT interval variability, but not QT prolongation per se, predicts drug-induced torsades de pointes in the anaesthetised methoxamine-sensitized rabbit.
[Jacobson Ingemar,Carlsson Leif,Duker Göran]J Pharmacol Toxicol Methods,Jan-Feb 2011;63(1):40-6. PMID: 20451633
111: Late na(+) current inhibition by ranolazine reduces torsades de pointes in the chronic atrioventricular block dog model.
[Antoons Gudrun,Oros Avram,Beekman Jet D M,Engelen Markus A,Houtman Marien J C,Belardinelli Luiz,Stengl Milan,Vos Marc A]J Am Coll Cardiol,2010 Feb 23;55(8):801-9. PMID: 20170820
112: Altered cytosolic Ca2+ dynamics in cultured Guinea pig cardiomyocytes as an in vitro model to identify potential cardiotoxicants.
[Qian Jian-Yong,Guo Liang]Toxicol In Vitro,2010 Apr;24(3):960-72. PMID: 20064605
113: Dofetilide is safe and effective in preventing atrial fibrillation recurrences in patients accepted for catheter ablation.
[Shamiss Yana,Khaykin Yaariv,Oosthuizen Richard,Tunney Denise,Sarak Bradley,Beardsall Marianne,Seabrook Catherine,Frost Linda,Wulffhart Zaev,Tsang Bernice,Verma Atul]Europace,2009 Nov;11(11):1448-55. PMID: 19819878
114: Initial experience with antiarrhythmic medication monitoring by clinical pharmacists in an outpatient setting: a retrospective review.
[Snider Melissa,Kalbfleisch Steven,Carnes Cynthia A]Clin Ther,2009 Jun;31(6):1209-18. PMID: 19695388
115: Dofetilide for atrial arrhythmias in congenital heart disease: a multicenter study.
[Wells Ronald,Khairy Paul,Harris Louise,Anderson C Christian,Balaji Seshadri]Pacing Clin Electrophysiol,2009 Oct;32(10):1313-8. PMID: 19691680
116: Models of torsades de pointes: effects of FPL64176, DPI201106, dofetilide, and chromanol 293B in isolated rabbit and guinea pig hearts.
[Cheng Hsien C,Incardona Josephine]J Pharmacol Toxicol Methods,Sep-Oct 2009;60(2):174-84. PMID: 19524054
117: Intravenous magnesium sulfate enhances the ability of dofetilide to successfully cardiovert atrial fibrillation or flutter: results of the Dofetilide and Intravenous Magnesium Evaluation.
[Coleman Craig I,Sood Nitesh,Chawla Dhruva,Talati Ripple,Ghatak Abhijit,Kluger Jeffrey,Dofetilide and Intravenous Magnesium Evaluation (DIME) Investigators]Europace,2009 Jul;11(7):892-5. PMID: 19351630
118: The role of the Na+/Ca2+ exchanger, I(Na) and I(CaL) in the genesis of dofetilide-induced torsades de pointes in isolated, AV-blocked rabbit hearts.
[Farkas Attila S,Makra Péter,Csík Norbert,Orosz Szabolcs,Shattock Michael J,Fülöp Ferenc,Forster Tamás,Csanády Miklós,Papp Julius Gy,Varró András,Farkas András]Br J Pharmacol,2009 Mar;156(6):920-32. PMID: 19222480
119: Calculation of QT shift in non clinical safety pharmacology studies.
[Champeroux Pascal,Martel Eric,Fowler John Sinclair Lawrence,Maurin Anne,Sola Marie Laure,Jude Sébastien,Elamrani Francine,Weyn Andrée Anne,Laveissiere Arnaud,Lala Patricia,Richard Serge]J Pharmacol Toxicol Methods,Mar-Apr 2009;59(2):73-85. PMID: 19135537
120: Safety of the novel atrial-selective K+-channel blocker AVE0118 in experimental heart failure.
[Schneider H-J,Husser O,Rihm M,Fredersdorf S,Birner C,Dhein S,Muders F,Jeron A,Goegelein H,Riegger G A,Luchner A]Naunyn Schmiedebergs Arch Pharmacol,2009 Mar;379(3):225-32. PMID: 18972103
121: Relationship among amiodarone, new class III antiarrhythmics, miscellaneous agents and acquired long QT syndrome.
[Riera Andrés Ricardo Pérez,Uchida Augusto Hiroshi,Ferreira Celso,Ferreira Filho Celso,Schapachnik Edgardo,Dubner Sergio,Zhang Li,Moffa Paulo Jorge]Cardiol J,2008;15(3):209-19. PMID: 18651412
122: An in vitro model for assessment of drug-induced torsade de pointes arrhythmia : effects of haloperidol and dofetilide on potential duration, repolarization inhomogeneities, and torsade de pointes arrhythmia.
[Dhein Stefan,Perlitz Franziska,Mohr Friedrich-Wilhelm]Naunyn Schmiedebergs Arch Pharmacol,2008 Dec;378(6):631-44. PMID: 18648775
123: Predicting drug-induced changes in QT interval and arrhythmias: QT-shortening drugs point to gaps in the ICHS7B Guidelines.
[Lu H R,Vlaminckx E,Hermans A N,Rohrbacher J,Van Ammel K,Towart R,Pugsley M,Gallacher D J]Br J Pharmacol,2008 Aug;154(7):1427-38. PMID: 18493243
124: Antiarrhythmic properties of a rapid delayed-rectifier current activator in rabbit models of acquired long QT syndrome.
[Diness Thomas G,Yeh Yung-Hsin,Qi Xiao Yan,Chartier Denis,Tsuji Yukiomi,Hansen Rie S,Olesen Soren-Peter,Grunnet Morten,Nattel Stanley]Cardiovasc Res,2008 Jul 1;79(1):61-9. PMID: 18367457
125: Safety considerations in the pharmacological management of atrial fibrillation.
[Camm A John]Int J Cardiol,2008 Jul 21;127(3):299-306. PMID: 18191470
126: Acute conversion of persistent atrial fibrillation during dofetilide initiation.
[Cotiga Delia,Arshad Aysha,Aziz Emad,Joshi Sandeep,Koneru Jayanthi N,Steinberg Jonathan S]Pacing Clin Electrophysiol,2007 Dec;30(12):1527-30. PMID: 18070309
127: The additive effects of the active component of grapefruit juice (naringenin) and antiarrhythmic drugs on HERG inhibition.
[Lin Congrong,Ke Xiaogang,Ranade Vasant,Somberg John]Cardiology,2008;110(3):145-52. PMID: 18057881
128: Measurements of blood pressure and electrocardiogram in conscious freely moving guineapigs: a model for screening QT interval prolongation effects.
[Hess P,Rey M,Wanner D,Steiner B,Clozel M]Lab Anim,2007 Oct;41(4):470-80. PMID: 17988441
129: Monotherapy versus combination therapy with class III antiarrhythmic agents to attenuate transmural dispersion of repolarization: a potential risk factor for torsade de pointes.
[Shah Sachin A,Kluger Jeffrey,White C Michael]Pharmacotherapy,2007 Sep;27(9):1297-305. PMID: 17723083
130: Risk factors and predictors of Torsade de pointes ventricular tachycardia in patients with left ventricular systolic dysfunction receiving Dofetilide.
[Pedersen Henriette Sloth,Elming Hanne,Seibaek Marie,Burchardt Hans,Brendorp Bente,Torp-Pedersen Christian,Køber Lars,DIAMOND Study Group]Am J Cardiol,2007 Sep 1;100(5):876-80. PMID: 17719337
131: Dofetilide-induced long QT and torsades de pointes.
[Aktas Mehmet K,Shah Abrar H,Akiyama Toshio]Ann Noninvasive Electrocardiol,2007 Jul;12(3):197-202. PMID: 17617063
132: Comparison of guinea-pig ventricular myocytes and dog Purkinje fibres for in vitro assessment of drug-induced delayed repolarization.
[Terrar Derek A,Wilson C M,Graham S G,Bryant S M,Heath B M]J Pharmacol Toxicol Methods,Sep-Oct 2007;56(2):171-85. PMID: 17596973
133: Action potential and QT prolongation not sufficient to cause Torsade de Pointes: role of action potential triangulation.
[Grant Augustus O,Tranquillo Joseph]J Cardiovasc Electrophysiol,2007 Feb;18(2):204-5. PMID: 17338768
134: Management of dofetilide overdose in a patient with known cocaine abuse.
[Campbell Kristen Bova,Mando Jennifer D,Gray Alice L,Robinson Eric]Pharmacotherapy,2007 Mar;27(3):459-63. PMID: 17316157
135: Frequency-dependent effects of various IKr blockers on cardiac action potential duration in a human atrial model.
[Tsujimae Kenji,Suzuki Shingo,Murakami Shingo,Kurachi Yoshihisa]Am J Physiol Heart Circ Physiol,2007 Jul;293(1):H660-9. PMID: 17220183
136: L-type calcium current reactivation contributes to arrhythmogenesis associated with action potential triangulation.
[Guo Donglin,Zhao Xiaojing,Wu Ying,Liu Tengxian,Kowey Peter R,Yan Gan-Xin]J Cardiovasc Electrophysiol,2007 Feb;18(2):196-203. PMID: 17212595
137: Use of a failing rabbit heart as a model to predict torsadogenicity.
[Kijtawornrat Anusak,Nishijima Yoshinori,Roche Brian M,Keene Bruce W,Hamlin Robert L]Toxicol Sci,2006 Sep;93(1):205-12. PMID: 16740615
138: Importance of extracardiac alpha1-adrenoceptor stimulation in assisting dofetilide to induce torsade de pointes in rabbit hearts.
[Farkas Attila S,Acsai Károly,Tóth András,Dézsi László,Orosz Szabolcs,Forster Tamás,Csanády Miklós,Papp Julius Gy,Varró András,Farkas András]Eur J Pharmacol,2006 May 10;537(1-3):118-25. PMID: 16618484
139: Use of arterially perfused rabbit ventricular wedge in predicting arrhythmogenic potentials of drugs.
[Chen Xian,Cordes Jason S,Bradley Jenifer A,Sun Zhuoqian,Zhou Jun]J Pharmacol Toxicol Methods,Nov-Dec 2006;54(3):261-72. PMID: 16564186
140: Isolated perfused and paced guinea pig heart to test for drug-induced changes of the QT interval.
[Cheng Hsien C,Incardona Josephine,McCullough Bruce]J Pharmacol Toxicol Methods,Nov-Dec 2006;54(3):278-87. PMID: 16507347
141: Evaluation of the rubidium efflux assay for preclinical identification of HERG blockade.
[Chaudhary Khuram W,O'Neal Janet M,Mo Zun-Li,Fermini Bernard,Gallavan Robert H,Bahinski Anthony]Assay Drug Dev Technol,2006 Feb;4(1):73-82. PMID: 16506891
142: Potassium channel subunit remodeling in rabbits exposed to long-term bradycardia or tachycardia: discrete arrhythmogenic consequences related to differential delayed-rectifier changes.
[Tsuji Yukiomi,Zicha Stephen,Qi Xiao-Yan,Kodama Itsuo,Nattel Stanley]Circulation,2006 Jan 24;113(3):345-55. PMID: 16432066
143: Assessment of drug-induced QT interval prolongation in conscious rabbits.
[Kijtawornrat A,Ozkanlar Y,Keene B W,Roche B M,Hamlin D M,Hamlin R L]J Pharmacol Toxicol Methods,Mar-Apr 2006;53(2):168-73. PMID: 16290300
144: Marked QT prolongation and torsades de pointes secondary to acute ischemia in an elderly man taking dofetilide for atrial fibrillation: a cautionary tale.
[Nagra Bipinpreet S,Ledley Gary S,Kantharia Bharat K]J Cardiovasc Pharmacol Ther,2005 Sep;10(3):191-5. PMID: 16211208
145: QT prolongation and proarrhythmia by moxifloxacin: concordance of preclinical models in relation to clinical outcome.
[Chen Xian,Cass Jessica D,Bradley Jenifer A,Dahm Corinn M,Sun Zhuoqian,Kadyszewski Edmund,Engwall Michael J,Zhou Jun]Br J Pharmacol,2005 Nov;146(6):792-9. PMID: 16158069
146: Restricting excessive cardiac action potential and QT prolongation: a vital role for IKs in human ventricular muscle.
[Jost Norbert,Virág László,Bitay Miklós,Takács János,Lengyel Csaba,Biliczki Péter,Nagy Zsolt,Bogáts Gábor,Lathrop David A,Papp Julius G,Varró András]Circulation,2005 Sep 6;112(10):1392-9. PMID: 16129791
147: Atypical proarrhythmia with dofetilide: monomorphic VT and exercise-induced torsade de pointes.
[Reiffel James A]Pacing Clin Electrophysiol,2005 Aug;28(8):877-9. PMID: 16105020
148: [Long QT syndrome].
[Watanabe Atsuyuki,Nakamura Kazufumi,Morita Hiroshi,Kusano Kengo Fukushima,Ohe Tohru]Nihon Rinsho,2005 Jul;63(7):1171-7. PMID: 16001778
149: Novel potent human ether-a-go-go-related gene (hERG) potassium channel enhancers and their in vitro antiarrhythmic activity.
[Zhou Jun,Augelli-Szafran Corinne E,Bradley Jenifer A,Chen Xian,Koci Bryan J,Volberg Walter A,Sun Zhuoqian,Cordes Jason S]Mol Pharmacol,2005 Sep;68(3):876-84. PMID: 15976038
150: A pharmacokinetic-pharmacodynamic model for the quantitative prediction of dofetilide clinical QT prolongation from human ether-a-go-go-related gene current inhibition data.
[Jonker Daniël M,Kenna Leslie A,Leishman Derek,Wallis Rob,Milligan Peter A,Jonsson E Niclas]Clin Pharmacol Ther,2005 Jun;77(6):572-82. PMID: 15961988
151: A new method to calculate the beat-to-beat instability of QT duration in drug-induced long QT in anesthetized dogs.
[van der Linde H,Van de Water A,Loots W,Van Deuren B,Lu H R,Van Ammel K,Peeters M,Gallacher D J]J Pharmacol Toxicol Methods,Jul-Aug 2005;52(1):168-77. PMID: 15935709
152: Comparative pharmacology of guinea pig cardiac myocyte and cloned hERG (I(Kr)) channel.
[Davie Christina,Pierre-Valentin Jean,Pollard Chris,Standen Nick,Mitcheson John,Alexander Paul,Thong Bob]J Cardiovasc Electrophysiol,2004 Nov;15(11):1302-9. PMID: 15574182
153: The [3H]dofetilide binding assay is a predictive screening tool for hERG blockade and proarrhythmia: Comparison of intact cell and membrane preparations and effects of altering [K+]o.
[Diaz Gilbert J,Daniell Katina,Leitza Sandra T,Martin Ruth L,Su Zhi,McDermott Jeffrey S,Cox Bryan F,Gintant Gary A]J Pharmacol Toxicol Methods,Nov-Dec 2004;50(3):187-99. PMID: 15519905
154: A benefit-risk assessment of class III antiarrhythmic agents.
[Elming Hanne,Brendorp Bente,Pehrson Steen,Pedersen Ole Dyg,Køber Lars,Torp-Petersen Christian]Expert Opin Drug Saf,2004 Nov;3(6):559-77. PMID: 15500415
155: IKr channel blockers: novel antiarrhythmic agents.
[Lee K,Park J Y,Ryu P D,Kwon L S,Kim H Y]Curr Med Chem Cardiovasc Hematol Agents,2003 Oct;1(3):203-23. PMID: 15326913
156: Torsade de pointes: the clinical considerations.
[Gowda Ramesh M,Khan Ijaz A,Wilbur Sabrina L,Vasavada Balendu C,Sacchi Terrence J]Int J Cardiol,2004 Jul;96(1):1-6. PMID: 15203254
157: Phase IV trial evaluating the effectiveness and safety of dofetilide.
[Guanzon Anna V,Crouch Michael A]Ann Pharmacother,Jul-Aug 2004;38(7-8):1142-7. PMID: 15161945
158: Clinical experience with dofetilide in the treatment of patients with atrial fibrillation.
[Prystowsky Eric N,Freeland Scott,Branyas Nancy A,Rardon David P,Fogel Richard I,Padanilam Benzy J,Rippy Janet S]J Cardiovasc Electrophysiol,2003 Dec;14(12 Suppl):S287-90. PMID: 15005216
159: Role of dofetilide in patients with atrial fibrillation. Insights from the Symptomatic Atrial Fibrillation Investigative Research on Dofetilide (SAFIRE-D) study.
[Singh Steven N]Card Electrophysiol Rev,2003 Sep;7(3):225-8. PMID: 14739718
160: Cardiac ion channel effects of tolterodine.
[Kang Jiesheng,Chen Xiao-Liang,Wang Hongge,Ji Junzhi,Reynolds William,Lim Sungtaek,Hendrix James,Rampe David]J Pharmacol Exp Ther,2004 Mar;308(3):935-40. PMID: 14711935
161: Electrophysiological safety of sertindole in dogs with normal and remodeled hearts.
[Thomsen Morten B,Volders Paul G A,Stengl Milan,Spätjens Roel L H M G,Beekman Jet D M,Bischoff Ulrike,Kall Morten A,Frederiksen Kristen,Matz Jørgen,Vos Marc A]J Pharmacol Exp Ther,2003 Nov;307(2):776-84. PMID: 12966159
162: Asynchronous development of electrical remodeling and cardiac hypertrophy in the complete AV block dog.
[Schoenmakers Marieke,Ramakers Christian,van Opstal Jurren M,Leunissen Jet D M,Londoño Camila,Vos Marc A]Cardiovasc Res,2003 Aug 1;59(2):351-9. PMID: 12909318
163: Dofetilide: A new antiarrhythmic agent approved for conversion and/or maintenance of atrial fibrillation/atrial flutter.
[Lenz T L,Hilleman D E]Drugs Today (Barc),2000 Nov;36(11):759-71. PMID: 12845335
164: A benefit-risk assessment of class III antiarrhythmic agents.
[Brendorp Bente,Pedersen Oledyg,Torp-Pedersen Christian,Sahebzadah Naji,Køber Lars]Drug Saf,2002;25(12):847-65. PMID: 12241126
165: Myocardial repolarization and drugs. Impossibility to predict the dominance of anti-arrhythmic over pro-arrhythmic effects of drugs due to differential and ventricular electrical remodeling.
[Vos M A,Crijns H J]Anadolu Kardiyol Derg,2001 Mar;1(1):27-34. PMID: 12122969
166: A multicentre, double-blind randomized crossover comparative study on the efficacy and safety of dofetilide vs sotalol in patients with inducible sustained ventricular tachycardia and ischaemic heart disease.
[Boriani G,Lubinski A,Capucci A,Niederle R,Kornacewicz-Jack Z,Wnuk-Wojnar A M,Borggrefe M,Brachmann J,Biffi M,Butrous G S,Ventricular Arrhythmias Dofetilide Investigators]Eur Heart J,2001 Dec;22(23):2180-91. PMID: 11913480
167: Electrophysiological mechanism of enhanced susceptibility of hypertrophied heart to acquired torsade de pointes arrhythmias: tridimensional mapping of activation and recovery patterns.
[Kozhevnikov Dmitry O,Yamamoto Keiji,Robotis Dionyssios,Restivo Mark,El-Sherif Nabil]Circulation,2002 Mar 5;105(9):1128-34. PMID: 11877367
168: Dofetilide in patients with congestive heart failure and left ventricular dysfunction: safety aspects and effect on atrial fibrillation. The Danish Investigators of Arrhythmia and Mortality on Dofetilide (DIAMOND) Study Group.
[Møller M,Torp-Pedersen C T,Køber L]Congest Heart Fail,May-Jun 2001;7(3):146-150. PMID: 11828153
169: Practical approach to the use and monitoring of dofetilide therapy.
[Tran A,Vichiendilokkul A,Racine E,Milad A]Am J Health Syst Pharm,2001 Nov 1;58(21):2050-9. PMID: 11715827
170: Importance of QT interval determination and renal function assessment during antiarrhythmic drug therapy.
[Reiffel J A,Appel G]J Cardiovasc Pharmacol Ther,2001 Apr;6(2):111-9. PMID: 11509917
171: Impact of sex and gonadal steroids on prolongation of ventricular repolarization and arrhythmias induced by I(K)-blocking drugs.
[Pham T V,Sosunov E A,Gainullin R Z,Danilo P,Rosen M R]Circulation,2001 May 1;103(17):2207-12. PMID: 11331264
172: Transgenic mice overexpressing human KvLQT1 dominant-negative isoform. Part II: Pharmacological profile.
[Lande G,Demolombe S,Bammert A,Moorman A,Charpentier F,Escande D]Cardiovasc Res,2001 May;50(2):328-34. PMID: 11334836
173: Pause-dependent polymorphic ventricular tachycardia during long-term treatment with dofetilide: a placebo-controlled, implantable cardioverter-defibrillator-based evaluation.
[Mazur A,Anderson M E,Bonney S,Roden D M]J Am Coll Cardiol,2001 Mar 15;37(4):1100-5. PMID: 11263615
174: Dofetilide: a new class III antiarrhythmic agent.
[Al-Dashti R,Sami M]Can J Cardiol,2001 Jan;17(1):63-7. PMID: 11173316
175: Azimilide and dofetilide produce similar electrophysiological and proarrhythmic effects in a canine model of Torsade de Pointes arrhythmias.
[Van Opstal J M,Leunissen J D,Wellens H J,Vos M A]Eur J Pharmacol,2001 Jan 19;412(1):67-76. PMID: 11166738
176: Effect of dofetilide in patients with recent myocardial infarction and left-ventricular dysfunction: a randomised trial.
[Køber L,Bloch Thomsen P E,Møller M,Torp-Pedersen C,Carlsen J,Sandøe E,Egstrup K,Agner E,Videbaek J,Marchant B,Camm A J,Danish Investigations of Arrhythmia and Mortality on Dofetilide (DIAMOND) Study Group]Lancet,2000 Dec 16;356(9247):2052-8. PMID: 11145491
177: Efficacy and safety of oral dofetilide in converting to and maintaining sinus rhythm in patients with chronic atrial fibrillation or atrial flutter: the symptomatic atrial fibrillation investigative research on dofetilide (SAFIRE-D) study.
[Singh S,Zoble R G,Yellen L,Brodsky M A,Feld G K,Berk M,Billing C B]Circulation,2000 Nov 7;102(19):2385-90. PMID: 11067793
178: Dofetilide: a class III anti-arrhythmic drug for the treatment of atrial fibrillation.
[Torp-Pedersen C,Brendorp B,Køber L]Expert Opin Investig Drugs,2000 Nov;9(11):2695-704. PMID: 11060831
179: Comparison of intravenously administered dofetilide versus amiodarone in the acute termination of atrial fibrillation and flutter. A multicentre, randomized, double-blind, placebo-controlled study.
[Bianconi L,Castro A,Dinelli M,Alboni P,Pappalardo A,Richiardi E,Santini M]Eur Heart J,2000 Aug;21(15):1265-73. PMID: 10924317
180: [Antiarrhythmic therapy in patients with heart failure].
[Faber T S,Zehender M]Ther Umsch,2000 May;57(5):324-32. PMID: 10859993
181: Drug choices in the treatment of atrial fibrillation.
[Reiffel J A]Am J Cardiol,2000 May 25;85(10A):12D-19D. PMID: 10822036
182: Steady-state versus non-steady-state QT-RR relationships in 24-hour Holter recordings.
[Lande G,Funck-Brentano C,Ghadanfar M,Escande D]Pacing Clin Electrophysiol,2000 Mar;23(3):293-302. PMID: 10750127
183: Dofetilide: a class III-specific antiarrhythmic agent.
[Kalus J S,Mauro V F]Ann Pharmacother,2000 Jan;34(1):44-56. PMID: 10669186
184: Proarrhythmia of azimilide and other class III antiarrhythmic agents in the adrenergically stimulated rabbit.
[Brooks R R,Drexler A P,Maynard A E,Al-Khalidi H,Kostreva D R]Proc Soc Exp Biol Med,2000 Feb;223(2):183-9. PMID: 10654622
185: Dofetilide: a review of its use in atrial fibrillation and atrial flutter.
[McClellan K J,Markham A]Drugs,1999 Dec;58(6):1043-59. PMID: 10651390
186: Dofetilide in patients with congestive heart failure and left ventricular dysfunction. Danish Investigations of Arrhythmia and Mortality on Dofetilide Study Group.
[Torp-Pedersen C,Møller M,Bloch-Thomsen P E,Køber L,Sandøe E,Egstrup K,Agner E,Carlsen J,Videbaek J,Marchant B,Camm A J]N Engl J Med,1999 Sep 16;341(12):857-65. PMID: 10486417
187: Exaggerated QT prolongation after cardioversion of atrial fibrillation.
[Choy A M,Darbar D,Dell'Orto S,Roden D M]J Am Coll Cardiol,1999 Aug;34(2):396-401. PMID: 10440151
188: Efficacy and safety of intravenously administered dofetilide in acute termination of atrial fibrillation and flutter: a multicenter, randomized, double-blind, placebo-controlled trial. Danish Dofetilide in Atrial Fibrillation and Flutter Study Group.
[Nørgaard B L,Wachtell K,Christensen P D,Madsen B,Johansen J B,Christiansen E H,Graff O,Simonsen E H]Am Heart J,1999 Jun;137(6):1062-9. PMID: 10347332
189: Effects of class III antiarrhythmic agents in an in vitro rabbit model of spontaneous torsades de pointe.
[D'Alonzo A J,Zhu J L,Darbenzio R B]Eur J Pharmacol,1999 Mar 12;369(1):57-64. PMID: 10204682
190: Dynamic analysis of dofetilide-induced changes in ventricular repolarization.
[Lande G,Maison-Blanche P,Fayn J,Ghadanfar M,Coumel P,Funck-Brentano C]Clin Pharmacol Ther,1998 Sep;64(3):312-21. PMID: 9757155
191: Azimilide dihydrochloride, a novel antiarrhythmic agent.
[Karam R,Marcello S,Brooks R R,Corey A E,Moore A]Am J Cardiol,1998 Mar 19;81(6A):40D-46D. PMID: 9537222
192: Current treatment recommendations in antiarrhythmic therapy.
[Van Gelder I C,Brügemann J,Crijns H J]Drugs,1998 Mar;55(3):331-46. PMID: 9530541
193: Electrophysiology and pharmacology of ibutilide.
[Naccarelli G V,Lee K S,Gibson J K,VanderLugt J]Am J Cardiol,1996 Oct 17;78(8A):12-6. PMID: 8903270
194: Influence of dofetilide on QT-interval duration and dispersion at various heart rates during exercise in humans.
[Démolis J L,Funck-Brentano C,Ropers J,Ghadanfar M,Nichols D J,Jaillon P]Circulation,1996 Oct 1;94(7):1592-9. PMID: 8840849
195: Differential effects of antiarrhythmic agents on post-pause repolarization in cardiac Purkinje fibres.
[Wyse K R,Bursill J A,Campbell T J]Clin Exp Pharmacol Physiol,1996 Sep;23(9):825-9. PMID: 8911721
196: Extracellular potassium modulation of drug block of IKr. Implications for torsade de pointes and reverse use-dependence.
[Yang T,Roden D M]Circulation,1996 Feb 1;93(3):407-11. PMID: 8565156
197: Assessment of reverse use-dependent blocking actions of class III antiarrhythmic drugs by 24-hour Holter electrocardiography.
[Okada Y,Ogawa S,Sadanaga T,Mitamura H]J Am Coll Cardiol,1996 Jan;27(1):84-9. PMID: 8522715
198: Effects of the new class III antiarrhythmic drug dofetilide on the atrial and ventricular intracardiac monophasic action potential in patients with angina pectoris.
[Sedgwick M L,Dalrymple I,Rae A P,Cobbe S M]Eur Heart J,1995 Nov;16(11):1641-6. PMID: 8881860
199: Pharmacokinetic and pharmacodynamic modeling of the effects of oral and intravenous administrations of dofetilide on ventricular repolarization.
[Le Coz F,Funck-Brentano C,Morell T,Ghadanfar M M,Jaillon P]Clin Pharmacol Ther,1995 May;57(5):533-42. PMID: 7768076
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.