Search for drugs:
Typing the drug name to query
IVACAFTOR
DIR Classification
Classification:Ambiguous
Severity Score:1.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Cardiac Electrophysiology
- The effect of multiple doses of ivacaftor 150 mg and 450 mg twice daily on QTc interval was evaluated in a randomized, placebo- and active-controlled (moxifloxacin 400 mg) four-period crossover thorough QT study in 72 healthy subjects. In a study with demonstrated ability to detect small effects, the upper bound of the one-sided 95% confidence interval for the largest placebo adjusted, baseline-corrected QTc based on Fridericia's correction method (QTcF) was below 10 ms, the threshold for regulatory concern.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
0
24092
Other ADRs
0
38381587
Odds Ratio = N/A
Drug Property Information
ATC Code(s):
- R07AX02 - ivacaftor
- R07AX - Other respiratory system products
- R07A - OTHER RESPIRATORY SYSTEM PRODUCTS
- R07 - OTHER RESPIRATORY SYSTEM PRODUCTS
- R - RESPIRATORY SYSTEM
- R07AX30 - ivacaftor
- R07AX - Other respiratory system products
- R07A - OTHER RESPIRATORY SYSTEM PRODUCTS
- R07 - OTHER RESPIRATORY SYSTEM PRODUCTS
- R - RESPIRATORY SYSTEM
- R07AX31 - ivacaftor
- R07AX - Other respiratory system products
- R07A - OTHER RESPIRATORY SYSTEM PRODUCTS
- R07 - OTHER RESPIRATORY SYSTEM PRODUCTS
- R - RESPIRATORY SYSTEM
- R07AX32 - ivacaftor
- R07AX - Other respiratory system products
- R07A - OTHER RESPIRATORY SYSTEM PRODUCTS
- R07 - OTHER RESPIRATORY SYSTEM PRODUCTS
- R - RESPIRATORY SYSTEM
Active Ingredient:ivacaftor
Active Ingredient UNII:1Y740ILL1Z
Drugbank ID:DB08820
PubChem Compound:16220172
CTD ID:C545203
PharmGKB:PA165950341
CAS Number:873054-44-5
Dosage Form(s):granule; tablet, film coated
Route(s) Of Administrator:oral
Daily Dose:
- 300.0 mg/day R07AX02
Chemical Structure: 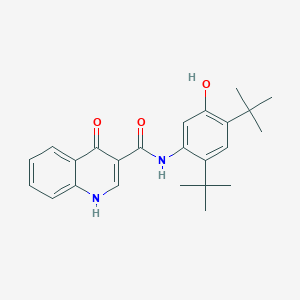

SMILE Code:
CC(C)(C)C1=CC(=C(O)C=C1NC(=O)C1=CNC2=CC=CC=C2C1=O)C(C)(C)C
CC(C)(C)C1=CC(=C(O)C=C1NC(=O)C1=CNC2=CC=CC=C2C1=O)C(C)(C)C
Reference
N/A
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.