Search for drugs:
Typing the drug name to query
FULVESTRANT
DIR Classification
Classification:Ambiguous
Severity Score:1.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- ADVERSE REACTIONS
- Clinical Trials Experience
- Additional adverse reactions in MONALEESA-3 for patients receiving Fulvestrant Injection plus ribociclib included asthenia (14%), dyspepsia (10%), thrombocytopenia (9%), dry skin (8%), dysgeusia (7%), electrocardiogram QT prolonged (6%), dry mouth (5%), vertigo (5%), dry eye (5%), lacrimation increased (4%), erythema (4%), hypocalcemia (4%), blood bilirubin increased (1%), and syncope (1%).
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
20
24072
Other ADRs
17825
38363762
Odds Ratio = 1.789
Drug Property Information
ATC Code(s):
- L02BA03 - fulvestrant
- L02BA - Anti-estrogens
- L02B - HORMONE ANTAGONISTS AND RELATED AGENTS
- L02 - ENDOCRINE THERAPY
- L - ANTINEOPLASTIC AND IMMUNOMODULATING AGENTS
Active Ingredient:FULVESTRANT
Active Ingredient UNII:22X328QOC4
Drugbank ID:DB00947
PubChem Compound:17756771
CTD ID:D000077267
PharmGKB:PA164747170
CAS Number:129453-61-8
Dosage Form(s):injection
Route(s) Of Administrator:intramuscular
Daily Dose:
- 8.3 mg/day L02BA03
Chemical Structure: 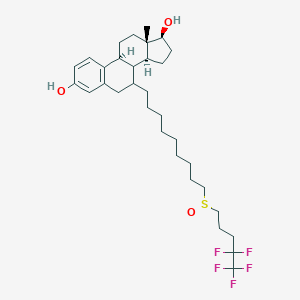

SMILE Code:
[H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])C3=CC=C(O)C=C3C[C@@H](CCCCCCCCCS(=O)CCCC(F)(F)C(F)(F)F)[C@@]21[H]
[H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])C3=CC=C(O)C=C3C[C@@H](CCCCCCCCCS(=O)CCCC(F)(F)C(F)(F)F)[C@@]21[H]
Reference
1: Real-world safety of palbociclib in breast cancer patients in the United States: a new user cohort study.
[Beachler Daniel C,de Luise Cynthia,Jamal-Allial Aziza,Yin Ruihua,Taylor Devon H,Suzuki Ayako,Lewis James H,Freston James W,Lanes Stephan]BMC Cancer,2021 Jan 25;21(1):97. PMID: 33494720
2: Ribociclib plus fulvestrant in the treatment of breast cancer.
[Neven Patrick,Sonke Gabe S,Jerusalem Guy]Expert Rev Anticancer Ther,2021 Jan;21(1):93-106. PMID: 33085548
3: Ribociclib in HR+/HER2- Advanced or Metastatic Breast Cancer Patients.
[Rascon Kaitlin,Flajc Goran,De Angelis Carmine,Liu Xinli,Trivedi Meghana V,Ekinci Ekim]Ann Pharmacother,2019 May;53(5):501-509. PMID: 30522347
4: Regional genomic regulation of cardiac sodium-calcium exchanger by oestrogen.
[Chen Guojun,Yang Xiaoyan,Alber Sean,Shusterman Vladimir,Salama Guy]J Physiol,2011 Mar 1;589(Pt 5):1061-80. PMID: 21224239
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.