Search for drugs:
Typing the drug name to query
ATOVAQUONE
DIR Classification
Classification:Ambiguous
Severity Score:1.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Cardiac Effects
- The effect of atovaquone oral suspension on the QT interval is unknown in humans.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
2
24090
Other ADRs
8040
38373547
Odds Ratio = 0.397
Drug Property Information
ATC Code(s):
- P01AX06 - atovaquone
- P01AX - Other agents against amoebiasis and other protozoal diseases
- P01A - AGENTS AGAINST AMOEBIASIS AND OTHER PROTOZOAL DISEASES
- P01 - ANTIPROTOZOALS
- P - "ANTIPARASITIC PRODUCTS, INSECTICIDES AND REPELLENTS"
Active Ingredient:ATOVAQUONE
Active Ingredient UNII:Y883P1Z2LT
Drugbank ID:DB01117
PubChem Compound:74989
CTD ID:D053626
PharmGKB:PA448502
CAS Number:95233-18-4
Dosage Form(s):suspension
Route(s) Of Administrator:oral
Daily Dose:
- 2250.0 mg/day P01AX06
Chemical Structure: 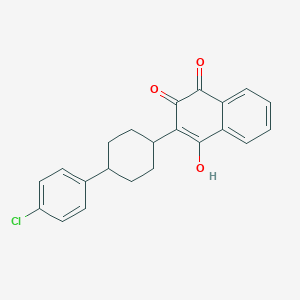

SMILE Code:
OC1=C([C@H]2CC[C@@H](CC2)C2=CC=C(Cl)C=C2)C(=O)C2=CC=CC=C2C1=O
OC1=C([C@H]2CC[C@@H](CC2)C2=CC=C(Cl)C=C2)C(=O)C2=CC=CC=C2C1=O
Reference
1: A pharmacovigilance study to quantify the strength of association between the combination of antimalarial drugs and azithromycin and cardiac arrhythmias: implications for the treatment of COVID-19.
[Diaby Vakaramoko,Almutairi Reem D,Chen Ziyan,Moussa Richard K,Berthe Abdrahmane]Expert Rev Pharmacoecon Outcomes Res,2021 Feb;21(1):159-168. PMID: 33186061
2: Drug interactions with antimalarial medications in older travelers: a clinical guide.
[Lewis Jelena,Gregorian Tania,Portillo Ivan,Goad Jeff]J Travel Med,2020 Feb 3;27(1):taz089. PMID: 31776555
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.