Search for drugs:
Typing the drug name to query
IXAZOMIB
DIR Classification
Classification:Ambiguous
Severity Score:1.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Cardiac Electrophysiology
- NINLARO did not prolong the QTc interval at clinically relevant exposures based on pharmacokinetic-pharmacodynamic analysis of data from 245 patients.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
6
24086
Other ADRs
24500
38357087
Odds Ratio = 0.391
Drug Property Information
ATC Code(s):
- L01XG03 - ixazomib
- L01XG -
- L01X - OTHER ANTINEOPLASTIC AGENTS
- L01 - ANTINEOPLASTIC AGENTS
- L - ANTINEOPLASTIC AND IMMUNOMODULATING AGENTS
Active Ingredient:ixazomib citrate
Active Ingredient UNII:46CWK97Z3K
Drugbank ID:DB09570
PubChem Compound:25183872
CTD ID: C548400
CAS Number:1072833-77-2
Dosage Form(s):capsule
Route(s) Of Administrator:oral
Daily Dose:
Chemical Structure: 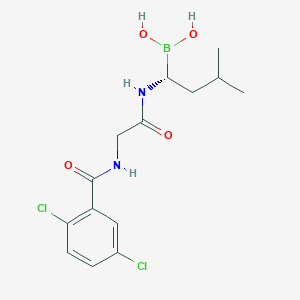

SMILE Code:
CC(C)C[C@H](NC(=O)CNC(=O)C1=CC(Cl)=CC=C1Cl)B(O)O
CC(C)C[C@H](NC(=O)CNC(=O)C1=CC(Cl)=CC=C1Cl)B(O)O
Reference
1: Integrated nonclinical and clinical risk assessment of the investigational proteasome inhibitor ixazomib on the QTc interval in cancer patients.
[Gupta Neeraj,Huh Yeamin,Hutmacher Matthew M,Ottinger Sean,Hui Ai-Min,Venkatakrishnan Karthik]Cancer Chemother Pharmacol,2015 Sep;76(3):507-16. PMID: 26141494
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.