Search for drugs:
Typing the drug name to query
RILPIVIRINE HYDROCHLORIDE
DIR Classification
Classification:Moderate-DIQT concern
Severity Score:3.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- WARNINGS AND PRECAUTIONS
- Risk of Adverse Reactions or Loss of Virologic Response Due to Drug Interactions
- The concomitant use of EDURANT and other drugs may result in potentially significant drug interactions, some of which may lead to [see DOSAGE AND ADMINISTRATION (2.2), CONTRAINDICATIONS (4), and DRUG INTERACTIONS (7)]:
- Loss of therapeutic effect of EDURANT and possible development of resistance.
- In healthy subjects, 75 mg once daily and 300 mg once daily (3 times and 12 times the dose in EDURANT) have been shown to prolong the QTc interval of the electrocardiogram. Consider alternatives to EDURANT when coadministered with a drug that is known to have a risk of torsade de pointes [see DRUG INTERACTIONS (7) and CLINICAL PHARMACOLOGY (12.2)].
- See TABLE 5 for steps to prevent or manage these possible and known significant drug interactions, including dosing recommendations. Consider the potential for drug interactions prior to and during EDURANT therapy and review concomitant medications during EDURANT therapy.
- DRUG INTERACTIONS
- QT Prolonging Drugs
- There is limited information available on the potential for a pharmacodynamic interaction between rilpivirine and drugs that prolong the QTc interval of the electrocardiogram. In a study of healthy subjects, 75 mg once daily and 300 mg once daily (3 times and 12 times the dose in EDURANT) have been shown to prolong the QTc interval of the electrocardiogram [see CLINICAL PHARMACOLOGY (12.2)]. Consider alternatives to EDURANT when coadministered with a drug with a known risk of torsade de pointes.
- OVERDOSAGE
- There is no specific antidote for overdose with EDURANT. Human experience of overdose with EDURANT is limited. Treatment of overdose with EDURANT consists of general supportive measures including monitoring of vital signs and ECG (QT interval) as well as observation of the clinical status of the patient. It is advisable to contact a poison control center to obtain the latest recommendations for the management of an overdose. Since rilpivirine is highly bound to plasma protein, dialysis is unlikely to result in significant removal of the active substance.
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Effects on Electrocardiogram
- The effect of EDURANT at the recommended dose of 25 mg once daily on the QTcF interval was evaluated in a randomized, placebo and active (moxifloxacin 400 mg once daily) controlled crossover study in 60 healthy adults, with 13 measurements over 24 hours at steady state. The maximum mean time-matched (95% upper confidence bound) differences in QTcF interval from placebo after baseline-correction was 2.0 (5.0) milliseconds (i.e., below the threshold of clinical concern).
- When doses of 75 mg once daily and 300 mg once daily of EDURANT (3 times and 12 times the dose in EDURANT) were studied in healthy adults, the maximum mean time-matched (95% upper confidence bound) differences in QTcF interval from placebo after baseline-correction were 10.7 (15.3) and 23.3 (28.4) milliseconds, respectively. Steady-state administration of EDURANT 75 mg once daily and 300 mg once daily resulted in a mean steady-state Cmax approximately 2.6-fold and 6.7-fold, respectively, higher than the mean Cmax observed with the recommended 25 mg once daily dose of EDURANT [see WARNINGS AND PRECAUTIONS (5.4)].
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
10
24082
Other ADRs
2014
38379573
Odds Ratio = 7.914
Drug Property Information
ATC Code(s):
- J05AG05 - rilpivirine hydrochloride
- J05AG - Non-nucleoside reverse transcriptase inhibitors
- J05A - DIRECT ACTING ANTIVIRALS
- J05 - ANTIVIRALS FOR SYSTEMIC USE
- J - ANTIINFECTIVES FOR SYSTEMIC USE
Active Ingredient:rilpivirine hydrochloride
Active Ingredient UNII:212WAX8KDD
Drugbank ID:DB08864
PubChem Compound:6451164
CTD ID:D000068696
CAS Number:500287-72-9
Dosage Form(s):tablet, film coated
Route(s) Of Administrator:oral
Daily Dose:
- 25.0 mg/day J05AG05
Chemical Structure: 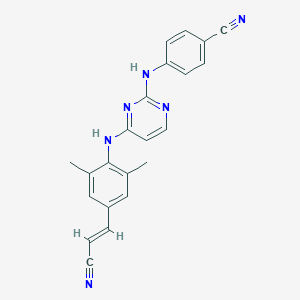

SMILE Code:
CC1=CC(\C=C\C#N)=CC(C)=C1NC1=CC=NC(NC2=CC=C(C=C2)C#N)=N1
CC1=CC(\C=C\C#N)=CC(C)=C1NC1=CC=NC(NC2=CC=C(C=C2)C#N)=N1
Reference
N/A
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.