Search for drugs:
Typing the drug name to query
CINACALCET
DIR Classification
Classification:Most-DIQT concern
Severity Score:4.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- WARNINGS AND PRECAUTIONS
- Hypocalcemia
- Cinacalcet hydrochloride tablets lowers serum calcium and can lead to hypocalcemia [see Adverse Reactions (6.1)]. Significant lowering of serum calcium can cause paresthesias, myalgias, muscle spasms, tetany, seizures, QT interval prolongation and ventricular arrhythmia. Life threatening events and fatal outcomes associated with hypocalcemia have been reported in patients treated with cinacalcet hydrochloride tablets, including in pediatric patients. The safety and effectiveness of cinacalcet hydrochloride tablets have not been established in pediatric patients [see Pediatric Use (8.4)].
- Cinacalcet hydrochloride tablets are not indicated for patients with CKD not on dialysis [see Indications and Usage (1)]. In patients with secondary HPT and CKD not on dialysis, the long term safety and efficacy of cinacalcet hydrochloride tablets have not been established. Clinical studies indicate that cinacalcet-treated patients with CKD not on dialysis have an increased risk for hypocalcemia compared with cinacalcet-treated patients with CKD on dialysis, which may be due to lower baseline calcium levels. In a phase 3 study of 32 weeks duration and including 404 patients with CKD not on dialysis (302 cinacalcet, 102 placebo), in which the median dose for cinacalcet was 60 mg per day at the completion of the study, 80% of cinacalcet-treated patients experienced at least one serum calcium value < 8.4 mg/dL compared with 5% of patients receiving placebo.
- QT Interval Prolongation and Ventricular Arrhythmia
- Decreases in serum calcium can also prolong the QT interval, potentially resulting in ventricular arrhythmia. Cases of QT prolongation and ventricular arrhythmia have been reported in patients treated with cinacalcet hydrochloride tablets.
- Patients with congenital long QT syndrome, history of QT interval prolongation, family history of long QT syndrome or sudden cardiac death, and other conditions that predispose to QT interval prolongation and ventricular arrhythmia may be at increased risk for QT interval prolongation and ventricular arrhythmias if they develop hypocalcemia due to cinacalcet hydrochloride. Closely monitor corrected serum calcium and QT interval in patients at risk receiving cinacalcet hydrochloride.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
40
24052
Other ADRs
61000
38320587
Odds Ratio = 1.045
Drug Property Information
ATC Code(s):
- H05BX01 - cinacalcet
- H05BX - Other anti-parathyroid agents
- H05B - ANTI-PARATHYROID AGENTS
- H05 - CALCIUM HOMEOSTASIS
- H - "SYSTEMIC HORMONAL PREPARATIONS, EXCL. "
Active Ingredient:CINACALCET HYDROCHLORIDE
Active Ingredient UNII:1K860WSG25
Drugbank ID:DB01012
PubChem Compound:156419
CTD ID:D000069449
PharmGKB:PA164776671
CAS Number:226256-56-0
Dosage Form(s):tablet
Route(s) Of Administrator:oral
Daily Dose:
- 60.0 mg/day H05BX01
Chemical Structure: 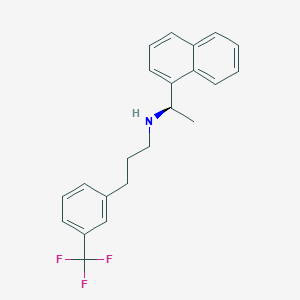

SMILE Code:
C[C@@H](NCCCC1=CC(=CC=C1)C(F)(F)F)C1=CC=CC2=CC=CC=C12
C[C@@H](NCCCC1=CC(=CC=C1)C(F)(F)F)C1=CC=CC2=CC=CC=C12
Reference
1: Effects of cinacalcet treatment on QT interval in hemodialysis patients.
[Temiz Gökhan,Yalçın Ahmet Uğur,Mutluay Rüya,Bozacı İlter,Bal Cengiz]Anatol J Cardiol,2016 Jul;16(7):520-523. PMID: 27004702
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.