Search for drugs:
Typing the drug name to query
SONIDEGIB
DIR Classification
Classification:Ambiguous
Severity Score:1.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Cardiac Electrophysiology
- At a dose of 800 mg once daily, sonidegib does not prolong the QTc interval.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
0
24092
Other ADRs
825
38380762
Odds Ratio = 0.0
Drug Property Information
ATC Code(s):
- L01XJ02 - sonidegib
- L01XJ -
- L01X - OTHER ANTINEOPLASTIC AGENTS
- L01 - ANTINEOPLASTIC AGENTS
- L - ANTINEOPLASTIC AND IMMUNOMODULATING AGENTS
Active Ingredient:SONIDEGIB PHOSPHATE
Active Ingredient UNII:W421AI34UW
Drugbank ID:DB09143
PubChem Compound:24775005
CTD ID: C561435
CAS Number:956697-53-3
Dosage Form(s):capsule
Route(s) Of Administrator:oral
Daily Dose:
Chemical Structure: 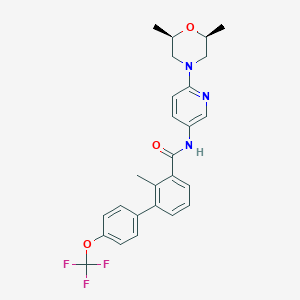

SMILE Code:
[H][C@]1(C)CN(C[C@@]([H])(C)O1)C1=CC=C(NC(=O)C2=CC=CC(=C2C)C2=CC=C(OC(F)(F)F)C=C2)C=N1
[H][C@]1(C)CN(C[C@@]([H])(C)O1)C1=CC=C(NC(=O)C2=CC=CC(=C2C)C2=CC=C(OC(F)(F)F)C=C2)C=N1
Reference
1: Exposure-QT analysis for sonidegib (LDE225), an oral inhibitor of the hedgehog signaling pathway, for measures of the QT prolongation potential in healthy subjects and in patients with advanced solid tumors.
[Quinlan Michelle,Zhou Jocelyn,Hurh Eunju,Sellami Dalila]Eur J Clin Pharmacol,2016 Dec;72(12):1427-1432. PMID: 27663457
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.