Search for drugs:
Typing the drug name to query
TRAMETINIB
DIR Classification
Classification:Ambiguous
Severity Score:1.0
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Cardiac Electrophysiology
- The heart rate-corrected QT (QTc) prolongation potential of trametinib was assessed in a dedicated study in 32 patients who received placebo on day 1 and MEKINIST 2 mg once daily on days 2-14 followed by MEKINIST 3 mg on day 15. No clinically relevant QTc prolongation was detected in the study.
- In clinical trials in patients who received MEKINIST with dabrafenib, QTc prolongation > 500 ms occurred in 0.8% of patients and QTc increased by > 60 ms from baseline in 3.8% of patients.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
QT Prolongation
15
24077
Other ADRs
15539
38366048
Odds Ratio = 1.539
Drug Property Information
ATC Code(s):
- L01EE01 - trametinib
- L01EE -
- L01E -
- L01 - ANTINEOPLASTIC AGENTS
- L - ANTINEOPLASTIC AND IMMUNOMODULATING AGENTS
- B01AC21 - trametinib
- B01AC - Platelet aggregation inhibitors excl. heparin
- B01A - ANTITHROMBOTIC AGENTS
- B01 - ANTITHROMBOTIC AGENTS
- B - BLOOD AND BLOOD FORMING ORGANS
Active Ingredient:TRAMETINIB DIMETHYL SULFOXIDE
Active Ingredient UNII:BSB9VJ5TUT
Drugbank ID:DB08911
PubChem Compound:11707110
CTD ID: C560077
PharmGKB:PA166115364
CAS Number:871700-17-3
Dosage Form(s):tablet, film coated
Route(s) Of Administrator:oral
Daily Dose:
- 4.3 mg/day B01AC21
Chemical Structure: 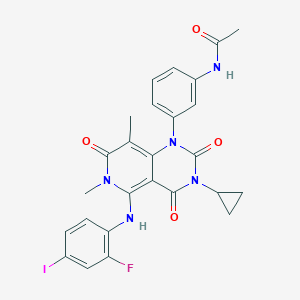

SMILE Code:
CN1C(=O)C(C)=C2N(C(=O)N(C3CC3)C(=O)C2=C1NC1=CC=C(I)C=C1F)C1=CC(NC(C)=O)=CC=C1
CN1C(=O)C(C)=C2N(C(=O)N(C3CC3)C(=O)C2=C1NC1=CC=C(I)C=C1F)C1=CC(NC(C)=O)=CC=C1
Reference
1: Cardiovascular Adverse Events Associated With BRAF and MEK Inhibitors: A Systematic Review and Meta-analysis.
[Mincu Raluca I,Mahabadi Amir A,Michel Lars,Mrotzek Simone M,Schadendorf Dirk,Rassaf Tienush,Totzeck Matthias]JAMA Netw Open,2019 Aug 2;2(8):e198890. PMID: 31397860
2: Tolerability of BRAF/MEK inhibitor combinations: adverse event evaluation and management.
[Heinzerling Lucie,Eigentler Thomas K,Fluck Michael,Hassel Jessica C,Heller-Schenck Daniela,Leipe Jan,Pauschinger Matthias,Vogel Arndt,Zimmer Lisa,Gutzmer Ralf]ESMO Open,2019 May 23;4(3):e000491. PMID: 31231568
3: Phase 1 study to evaluate the effect of the MEK inhibitor trametinib on cardiac repolarization in patients with solid tumours.
[Patnaik Amita,Tolcher Anthony,Papadopoulos Kyriakos P,Beeram Murali,Rasco Drew,Werner Theresa L,Bauman John W,Scheuber Anita,Cox Donna S,Patel Bela R,Zhou YanYan,Hamid Mohammed,Schramek Daniel,Sharma Sunil]Cancer Chemother Pharmacol,2016 Sep;78(3):491-500. PMID: 27392790
4: Update on Cardiovascular Safety of Tyrosine Kinase Inhibitors: With a Special Focus on QT Interval, Left Ventricular Dysfunction and Overall Risk/Benefit.
[Shah Rashmi R,Morganroth Joel]Drug Saf,2015 Aug;38(8):693-710. PMID: 26008987
Disclaimer:
The content of this database of QT prolongation is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
Any mention of commercial products is for clarification and not intended as endorsement.