Search for drugs:
Typing the drug name to query
AMIODARONE HYDROCHLORIDE
DIR Classification
Classification:Less-DIR concern
Severity Score:1
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- WARNINGS
- Proarrhythmia
- Like all antiarrhythmic agents, amiodarone HCl injection may cause a worsening of existing arrhythmias or precipitate a new arrhythmia. Proarrhythmia, primarily torsade de pointes (TdP), has been associated with prolongation by amiodarone HCl injection of the QTc interval to 500 ms or greater. Although QTc prolongation occurred frequently in patients receiving amiodarone HCl injection, torsade de pointes or new-onset VF occurred infrequently (less than 2%). Patients should be monitored for QTc prolongation during infusion with amiodarone HCl injection. Combination of amiodarone with other antiarrhythmic therapy that prolongs the QTc should be reserved for patients with life-threatening ventricular arrhythmias who are incompletely responsive to a single agent.
- Fluoroquinolones, macrolide antibiotics, and azoles are known to cause QTc prolongation. There have been reports of QTc prolongation, with or without TdP, in patients taking amiodarone when fluoroquinolones, macrolide antibiotics, or azoles were administered concomitantly (see DRUG INTERACTIONS, OTHER REPORTED INTERACTIONS WITH AMIODARONE).
- The need to coadminister amiodarone with any other drug known to prolong the QTc interval must be based on a careful assessment of the potential risks and benefits of doing so for each patient.
- A careful assessment of the potential risks and benefits of administering amiodarone HCl injection must be made in patients with thyroid dysfunction due to the possibility of arrhythmia breakthrough or exacerbation of arrhythmia, which may result in death, in these patients.
- PRECAUTIONS
- Drug Interactions
- Loratadine, a non-sedating antihistaminic, is metabolized primarily by CYP3A4. QT interval prolongation and torsade de pointes have been reported with the co-administration of loratadine and amiodarone.
- Trazodone, an antidepressant, is metabolized primarily by CYP3A4. QT interval prolongation and torsade de pointes have been reported with the co-administration of trazodone and amiodarone.
- Disopyramide increases QT prolongation which could cause arrhythmia.
- Fluoroquinolones, macrolide antibiotics, and azoles are known to cause QTc prolongation. There have been reports of QTc prolongation, with or without TdP, in patients taking amiodarone when fluoroquinolones, macrolide antibiotics, or azoles were administered concomitantly (see PRECAUTIONS, PROARRHYTHMIA).
- Electrolyte Disturbances
- Patients with hypokalemia or hypomagnesemia should have the condition corrected whenever possible before being treated with amiodarone HCl injection, as these disorders can exaggerate the degree of QTc prolongation and increase the potential for TdP. Special attention should be given to electrolyte and acid-base balance in patients experiencing severe or prolonged diarrhea or in patients receiving concomitant diuretics.
- ADVERSE REACTIONS
- Other treatment-emergent possibly drug-related adverse events reported in less than 2% of patients receiving amiodarone HCl injection in controlled and uncontrolled studies included the following: abnormal kidney function, atrial fibrillation, diarrhea, increased ALT, increased AST, lung edema, nodal arrhythmia, prolonged QT interval, respiratory disorder, shock, sinus bradycardia, Stevens-Johnson syndrome, thrombocytopenia, VF, and vomiting.
- CLINICAL PHARMACOLOGY
- Mechanisms of Action
- Amiodarone HCl Injection administration prolongs intranodal conduction (Atrial-His, AH) and refractoriness of the atrioventricular node (ERP AVN), but has little or no effect on sinus cycle length (SCL), refractoriness of the right atrium and right ventricle (ERP RA and ERP RV), repolarization (QTc), intraventricular conduction (QRS), and infranodal conduction (His-ventricular, HV). A comparison of the electrophysiologic effects of amiodarone HCl injection and oral amiodarone is shown in the table below.
- >>17a1383b-fdff-4054-8815-d5ef47d892e0.jpeg
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
140
42772
Other ADRs
14781
14102498
Odds Ratio = 3.123
Drug Property Information
ATC Code(s):
- C01BD01 - amiodarone hydrochloride
- C01BD - "Antiarrhythmics, class III"
- C01B - "ANTIARRHYTHMICS, CLASS I AND III"
- C01 - CARDIAC THERAPY
- C - CARDIOVASCULAR SYSTEM
Active Ingredient:amiodarone hydrochloride
Active Ingredient UNII:976728SY6Z
Drugbank ID:DB01118
PubChem Compound:2157
CAS Number:1951-25-3
Dosage Form(s):injection
Route(s) Of Administrator:intravenous
Daily Dose:
- 200.0 mg/day C01BD01
Chemical Structure: 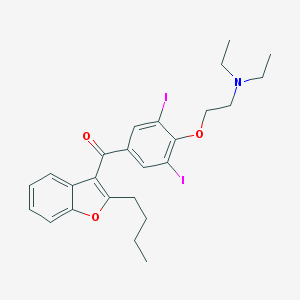

SMILE Code:
CCCCC1=C(C2=CC=CC=C2O1)C(=O)C3=CC(=C(C(=C3)I)OCCN(CC)CC)I
CCCCC1=C(C2=CC=CC=C2O1)C(=O)C3=CC(=C(C(=C3)I)OCCN(CC)CC)I
Reference
COHORT STUDY:
1: Assessment of statin-associated muscle toxicity in Japan: a cohort study conducted using claims database and laboratory information.
[Chang CH, Kusama M, Ono S, Sugiyama Y, Orii T, Akazawa M, BMJ Open. 2013 Apr 11;3(4).]ABSTRACT
OBJECTIVE: To estimate the incidence of muscle toxicity in patients receiving statin therapy by examining study populations, drug exposure status and outcome definitions.
DESIGN: A retrospective cohort study.
SETTING: 16 medical facilities in Japan providing information on laboratory tests performed in and claims received by their facilities between 1 April 2004 and 31 December 2010.
PARTICIPANTS: A database representing a cohort of 35 903 adult statin (atorvastatin, fluvastatin, pitavastatin, pravastatin, rosuvastatin and simvastatin) users was studied. Use of interacting drugs (fibrates, triazoles, macrolides, amiodarone and ciclosporin) by these patients was determined.
MAIN OUTCOME MEASURE: Statin-associated muscle toxicity (the 'event') was identified based on a diagnosis of muscle-related disorders (myopathy or rhabdomyolysis) and/or abnormal elevation of creatine kinase (CK) concentrations. Events were excluded if the patients had CK elevation-related conditions other than muscle toxicity. Incidence rates for muscle toxicity were determined per 1000 person-years, with 95% CI determined by Poisson regression.
RESULTS: A total of 18 036 patients accounted for 42 193 person-years of statin therapy, and 43 events were identified. The incidence of muscle toxicity in the patients treated with statins was 1.02 (95% CI 0.76 to 1.37)/1000 person-years. The estimates varied when outcome definitions were modified from 0.09/1000 person-years, which met both diagnosis and CK 10× greater than the upper limit of normal range (ULN) criteria, to 2.06/1000 person-years, which met diagnosis or CK 5× ULN criterion. The incidence of muscle toxicity was also influenced by the statin therapies selected, but no significant differences were observed. Among 2430 patients (13.5%) received interacting drugs with statins, only three muscle toxicity cases were observed (incidence: 1.69/1000 person-years).
CONCLUSIONS: This database study suggested that statin use is generally well tolerated and safe; however, the risk of muscle toxicity related to the use of interacting drugs requires further exploration.
PMID: 23585384
DESIGN: A retrospective cohort study.
SETTING: 16 medical facilities in Japan providing information on laboratory tests performed in and claims received by their facilities between 1 April 2004 and 31 December 2010.
PARTICIPANTS: A database representing a cohort of 35 903 adult statin (atorvastatin, fluvastatin, pitavastatin, pravastatin, rosuvastatin and simvastatin) users was studied. Use of interacting drugs (fibrates, triazoles, macrolides, amiodarone and ciclosporin) by these patients was determined.
MAIN OUTCOME MEASURE: Statin-associated muscle toxicity (the 'event') was identified based on a diagnosis of muscle-related disorders (myopathy or rhabdomyolysis) and/or abnormal elevation of creatine kinase (CK) concentrations. Events were excluded if the patients had CK elevation-related conditions other than muscle toxicity. Incidence rates for muscle toxicity were determined per 1000 person-years, with 95% CI determined by Poisson regression.
RESULTS: A total of 18 036 patients accounted for 42 193 person-years of statin therapy, and 43 events were identified. The incidence of muscle toxicity in the patients treated with statins was 1.02 (95% CI 0.76 to 1.37)/1000 person-years. The estimates varied when outcome definitions were modified from 0.09/1000 person-years, which met both diagnosis and CK 10× greater than the upper limit of normal range (ULN) criteria, to 2.06/1000 person-years, which met diagnosis or CK 5× ULN criterion. The incidence of muscle toxicity was also influenced by the statin therapies selected, but no significant differences were observed. Among 2430 patients (13.5%) received interacting drugs with statins, only three muscle toxicity cases were observed (incidence: 1.69/1000 person-years).
CONCLUSIONS: This database study suggested that statin use is generally well tolerated and safe; however, the risk of muscle toxicity related to the use of interacting drugs requires further exploration.
OTHER REFERENCE(S):
N/ADisclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.