Search for drugs:
Typing the drug name to query
AMPHOTERICIN B
DIR Classification
Classification:Less-DIR concern
Severity Score:1
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- ADVERSE REACTIONS
- Post-marketing Experience
- The following infrequent adverse experiences have been reported in post-marketing surveillance, in addition to those mentioned above: angioedema, erythema, urticaria, bronchospasm, cyanosis/hypoventilation, pulmonary edema, agranulocytosis, hemorrhagic cystitis, and rhabdomyolysis.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
120
42792
Other ADRs
4241
14113038
Odds Ratio = 9.332
Drug Property Information
ATC Code(s):
- J02AA01 - amphotericin b
- J02AA - Antibiotics
- J02A - ANTIMYCOTICS FOR SYSTEMIC USE
- J02 - ANTIMYCOTICS FOR SYSTEMIC USE
- J - ANTIINFECTIVES FOR SYSTEMIC USE
- A01AB04 - amphotericin b
- A01AB - Antiinfectives and antiseptics for local oral treatment
- A01A - STOMATOLOGICAL PREPARATIONS
- A01 - STOMATOLOGICAL PREPARATIONS
- A - ALIMENTARY TRACT AND METABOLISM
- A07AA07 - amphotericin b
- A07AA - Antibiotics
- A07A - INTESTINAL ANTIINFECTIVES
- A07 - "ANTIDIARRHEALS, INTESTINAL ANTIINFLAMMATORY/ANTIINFECTIVE "
- A - ALIMENTARY TRACT AND METABOLISM
- G01AA03 - amphotericin b
- G01AA - Antibiotics
- G01A - "ANTIINFECTIVES AND ANTISEPTICS, EXCL. COMBINATIONS "
- G01 - GYNECOLOGICAL ANTIINFECTIVES AND ANTISEPTICS
- G - GENITO URINARY SYSTEM AND SEX HORMONES
Active Ingredient:amphotericin b
Active Ingredient UNII:7XU7A7DROE
Drugbank ID:DB00681
PubChem Compound:5280965
CAS Number:1397-89-3
Dosage Form(s):injection, powder, lyophilized, for solution
Route(s) Of Administrator:intravenous
Daily Dose:
- 40.0 mg/day A01AB04
- 400.0 mg/day A07AA07
- 200.0 mg/day G01AA03
- 35.0 mg/day J02AA01
Chemical Structure: 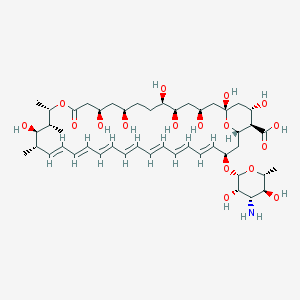

SMILE Code:
C[C@H]1/C=C/C=C/C=C/C=C/C=C/C=C/C=C/[C@@H](C[C@H]2[C@@H]([C@H](C[C@](O2)(C[C@H](C[C@H]([C@@H](CC[C@H](C[C@H](CC(=O)O[C@H]([C@@H]([C@@H]1O)C)C)O)O)O)O)O)O)O)C(=O)O)O[C@H]3[C@H]([C@H]([C@@H]([C@H](O3)C)O)N)O
C[C@H]1/C=C/C=C/C=C/C=C/C=C/C=C/C=C/[C@@H](C[C@H]2[C@@H]([C@H](C[C@](O2)(C[C@H](C[C@H]([C@@H](CC[C@H](C[C@H](CC(=O)O[C@H]([C@@H]([C@@H]1O)C)C)O)O)O)O)O)O)O)C(=O)O)O[C@H]3[C@H]([C@H]([C@@H]([C@H](O3)C)O)N)O
Reference
COHORT STUDY:
N/AOTHER REFERENCE(S):
1: Hypokalaemia-induced rhabdomyolysis after treatment of post-Kala-azar dermal Leishmaniasis (PKDL) with high-dose AmBisome in Bangladesh-a case report.
[Marking Ulrika,den Boer Margriet,Das Asish Kumar,Ahmed Elshafie Mohamed,Rollason Victoria,Ahmed Be-Nazir,Davidson Robert N,Ritmeijer Koert]PLoS Negl Trop Dis.2014 Jun 12;8(6):e2864. doi: 10.1371/journal.pntd.0002864. eCollection 2014 Jun. PMID: 24922279
2: Nephrotoxicity as a cause of acute kidney injury in children.
[Patzer Ludwig]Pediatr Nephrol.2008 Dec;23(12):2159-73. doi: 10.1007/s00467-007-0721-x. Epub 2008 Jan 29. PMID: 18228043
3: Absidia Corymbifera in an immune competent accident victim with multiple abdominal injuries: case report.
[Belfiori Rita,Terenzi Adelmo,Marchesini Laura,Repetto Antonella]BMC Infect Dis.2007 May 25;7:46. PMID: 17531089
4: Hypokalemic rhabdomyolysis in a child due to amphotericin B therapy.
[Lucas da Silva Paulo Sérgio,Iglesias Simone Brasil de Oliveira,Waisberg Jaques]Eur J Pediatr.2007 Feb;166(2):169-71. Epub 2006 Aug 12. PMID: 16906399
5: Severe rhabdomyolysis, hyperthermia and shock after amphotericin B colloidal dispersion in an allogeneic bone marrow transplant recipient.
[Rossi M R,Longoni D V,Rovelli A M,Uderzo C]Pediatr Infect Dis J.2000 Feb;19(2):172-3. PMID: 10694013
6: Disseminated zygomycosis due to Rhizopus schipperae after heatstroke.
[Anstead G M,Sutton D A,Thompson E H,Weitzman I,Otto R A,Ahuja S K]J Clin Microbiol.1999 Aug;37(8):2656-62. PMID: 10405417
7: The mechanism of muscle injury in the crush syndrome: ischemic versus pressure-stretch myopathy.
[Better O S,Abassi Z,Rubinstein I,Marom S,Winaver Y,Silberman M]Miner Electrolyte Metab.1990;16(4):181-4. PMID: 2277600
8: Hypokalemic rhabdomyolysis and myoglobinuria following amphotericin B therapy.
[Drutz D J,Fan J H,Tai T Y,Cheng J T,Hsieh W C]JAMA.1970 Feb 2;211(5):824-6. PMID: 5536569
Disclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.