Search for drugs:
Typing the drug name to query
ALBENDAZOLE
DIR Classification
Classification:Less-DIR concern
Severity Score:1
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- ADVERSE REACTIONS
- Postmarketing Experience
- The following adverse reactions have been identified during post-approval use of ALBENZA. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Blood and Lymphatic System Disorders: Aplastic anemia, bone marrow suppression, neutropenia.
- Eye Disorders: Vision blurred.
- Gastrointestinal Disorders: Diarrhea.
- General System Disorders: Asthenia.
- Hepatobiliary Disorders: Elevations of hepatic enzymes, hepatitis, acute liver failure.
- Musculoskeletal and Connective Tissue Disorders: Rhabdomyolysis.
- Nervous System Disorders: Somnolence, convulsion.
- Renal and Urinary Disorders: Acute renal failure.
- Skin and Subcutaneous Tissue Disorders: Erythema multiforme, Stevens-Johnson syndrome.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
1
42911
Other ADRs
713
14116566
Odds Ratio = 0.462
Drug Property Information
ATC Code(s):
- P02CA03 - albendazole
- P02CA - Benzimidazole derivatives
- P02C - ANTINEMATODAL AGENTS
- P02 - ANTHELMINTICS
- P - "ANTIPARASITIC PRODUCTS, INSECTICIDES AND REPELLENTS"
Active Ingredient:albendazole
Active Ingredient UNII:F4216019LN
Drugbank ID:DB00518
PubChem Compound:2082
CAS Number:54965-21-8
Dosage Form(s):tablet, chewable; tablet, film coated
Route(s) Of Administrator:oral
Daily Dose:
- 400.0 mg/day P02CA03
Chemical Structure: 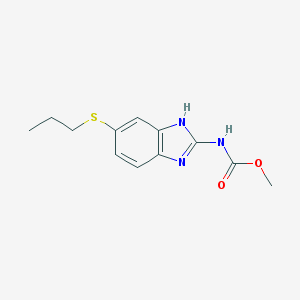

SMILE Code:
CCCSC1=CC2=C(C=C1)N=C(N2)NC(=O)OC
CCCSC1=CC2=C(C=C1)N=C(N2)NC(=O)OC
Reference
COHORT STUDY:
N/AOTHER REFERENCE(S):
1: [Hydatid disease of the femoral shaft treated with surgery and hypertonic solution wash-out combined with albendazole].
[Winter M,Jacquot N,Balaguer T,de Peretti F]Rev Chir Orthop Reparatrice Appar Mot.2005 Oct;91(6):564-8. PMID: 16327693
Disclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.