Search for drugs:
Typing the drug name to query
ACITRETIN
DIR Classification
Classification:Most-DIR concern
Severity Score:4
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- WARNINGS
- Capillary Leak Syndrome:
- Capillary leak syndrome, a potential manifestation of retinoic acid syndrome, has been reported in patients receiving acitretin. Features of this syndrome may include localized or generalized edema with secondary weight gain, fever, and hypotension. Rhabdomyolysis and myalgias have been reported in association with capillary leak syndrome, and laboratory tests may reveal neutrophilia, hypoalbuminemia, and an elevated hematocrit. Discontinue acitretin if capillary leak syndrome develops during therapy.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
12
42900
Other ADRs
2473
14114806
Odds Ratio = 1.597
Drug Property Information
ATC Code(s):
- D05BB02 - acitretin
- D05BB - Retinoids for treatment of psoriasis
- D05B - ANTIPSORIATICS FOR SYSTEMIC USE
- D05 - ANTIPSORIATICS
- D - DERMATOLOGICALS
Active Ingredient:acitretin
Active Ingredient UNII:LCH760E9T7
Drugbank ID:DB00459
PubChem Compound:5284513
CAS Number:55079-83-9
Dosage Form(s):capsule
Route(s) Of Administrator:oral
Daily Dose:
- 35.0 mg/day D05BB02
Chemical Structure: 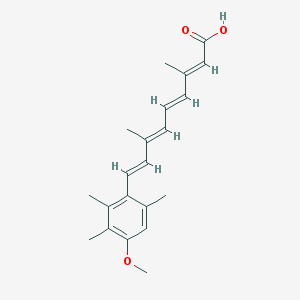

SMILE Code:
CC1=CC(=C(C(=C1/C=C/C(=C/C=C/C(=C/C(=O)O)/C)/C)C)C)OC
CC1=CC(=C(C(=C1/C=C/C(=C/C=C/C(=C/C(=O)O)/C)/C)C)C)OC
Reference
COHORT STUDY:
N/AOTHER REFERENCE(S):
1: Neuromuscular adverse effects associated with systemic retinoid dermatotherapy: monitoring and treatment algorithm for clinicians.
[Chroni Elisabeth,Monastirli Alexandra,Tsambaos Dionysios]Drug Saf.2010 Jan 1;33(1):25-34. doi: 10.2165/11319020-000000000-00000. PMID: 20000864
Disclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.