Search for drugs:
Typing the drug name to query
AVELUMAB
DIR Classification
Classification:Moderate-DIR concern
Severity Score:2
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- DOSAGE AND ADMINISTRATION
- Dose Modifications
- Recommended dose modifications of BAVENCIO for adverse reactions are provided in Table 1. Detailed information regarding clinical and laboratory monitoring guidelines for early detection of adverse reactions of BAVENCIO and recommended management (immunosuppressant treatment guidelines) are described in Warnings and Precautions (5).
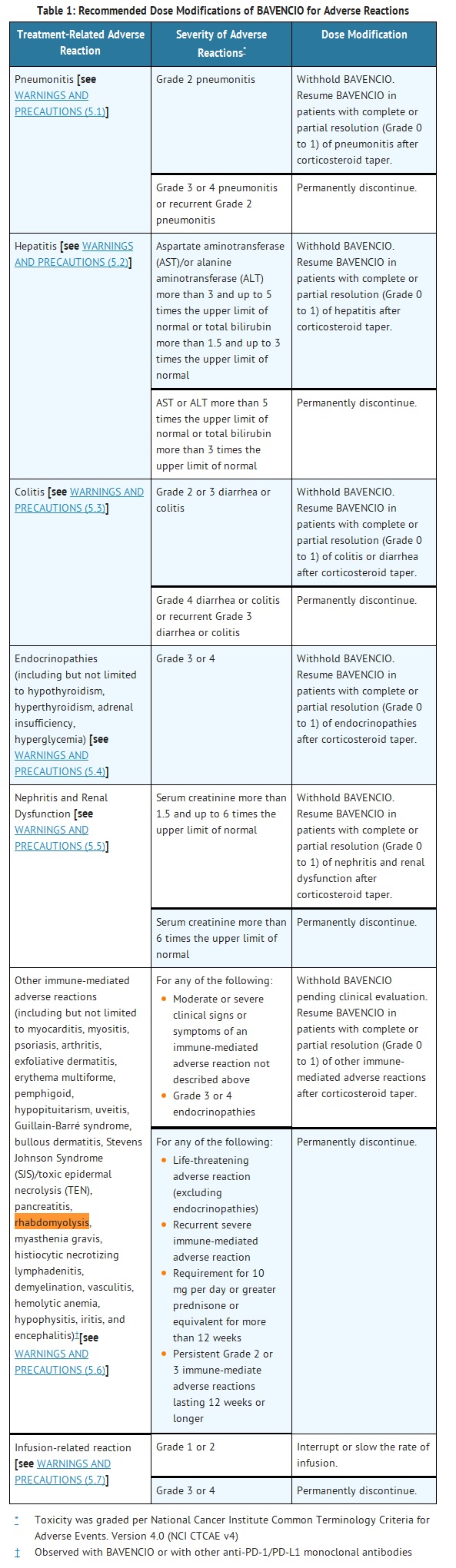
- WARNINGS AND PRECAUTIONS
- Other Immune-Mediated Adverse Reactions
- BAVENCIO can result in severe and fatal immune-mediated adverse reactions [see ADVERSE REACTIONS (6.1)]. These immune-mediated reactions may involve any organ system. Most immune-mediated reactions initially manifest during treatment with BAVENCIO; however, immune-mediated adverse reactions can occur after discontinuation of BAVENCIO.
- For suspected immune-mediated adverse reactions, evaluate to confirm or rule out an immune-mediated adverse reaction and to exclude other causes. Depending upon the severity of the adverse reaction, withhold or permanently discontinue BAVENCIO, administer high dose corticosteroids, and if appropriate, initiate hormone replacement therapy. Upon improvement to Grade 1 or less, initiate corticosteroid taper. Resume BAVENCIO when the immune-mediated adverse reaction remains at Grade 1 or less following corticosteroid taper. Permanently discontinue BAVENCIO for any severe (Grade 3) immune-mediated adverse reaction that recurs and for any life-threatening immune-mediated adverse reaction [see DOSAGE AND ADMINISTRATION (2.3)].
- The following clinically significant, immune-mediated adverse reactions occurred at an incidence of less than 1% of 1738 patients treated with BAVENCIO for each of the following adverse reactions: immune-mediated myocarditis including fatal cases, immune-mediated myositis, psoriasis, arthritis, exfoliative dermatitis, erythema multiforme, pemphigoid, hypopituitarism, uveitis, Guillain-Barré syndrome, and systemic inflammatory response. The following clinically significant, immune-mediated adverse reactions have been reported with other products in this class: bullous dermatitis, Stevens Johnson Syndrome (SJS)/toxic epidermal necrolysis (TEN), pancreatitis, rhabdomyolysis, myasthenia gravis, histiocytic necrotizing lymphadenitis, demyelination, vasculitis, hemolytic anemia, hypophysitis, iritis, and encephalitis.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
0
42912
Other ADRs
60
14117219
Odds Ratio = N/A
Drug Property Information
ATC Code(s):
N/A
Active Ingredient:avelumab
Active Ingredient UNII:KXG2PJ551I
Drugbank ID:DB11945
PubChem Compound:N/A
CAS Number:1537032-82-8
Dosage Form(s):injection, solution, concentrate
Route(s) Of Administrator:intravenous
Daily Dose:
- 20.0 mg/day L01
Chemical Structure: 

SMILE Code:
-
-
Reference
COHORT STUDY:
N/AOTHER REFERENCE(S):
N/ADisclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.