Search for drugs:
Typing the drug name to query
BUPROPION HYDROBROMIDE
DIR Classification
Classification:Moderate-DIR concern
Severity Score:2
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- ADVERSE REACTIONS
- Postmarketing Experience
- The following adverse reactions have been identified during post approval use of APLENZIN. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Body (General)
- Chills, facial edema, edema, peripheral edema, musculoskeletal chest pain, photosensitivity, and malaise.
- Cardiovascular
- Postural hypotension, stroke, vasodilation, syncope, complete atrioventricular block, extrasystoles, myocardial infarction, phlebitis, and pulmonary embolism.
- Digestive
- Abnormal liver function, bruxism, gastric reflux, gingivitis, glossitis, increased salivation, jaundice, mouth ulcers, stomatitis, thirst, edema of tongue, colitis, esophagitis, gastrointestinal hemorrhage, gum hemorrhage, hepatitis, intestinal perforation, liver damage, pancreatitis, and stomach ulcer.
- Endocrine
- Hyperglycemia, hypoglycemia, and syndrome of inappropriate antidiuretic hormone secretion.
- Hemic and Lymphatic
- Ecchymosis, anemia, leukocytosis, leukopenia, lymphadenopathy, pancytopenia, and thrombocytopenia. Altered PT and/or INR, associated with hemorrhagic or thrombotic complications, were observed when bupropion was coadministered with warfarin.
- Metabolic and Nutritional
- Glycosuria.
- Musculoskeletal
- Leg cramps, fever/rhabdomyolysis, and muscle weakness.
- Nervous System
- Abnormal coordination, depersonalization, emotional lability, hyperkinesia, hypertonia, hypesthesia, vertigo, amnesia, ataxia, derealization, abnormal electroencephalogram (EEG), aggression, akinesia, aphasia, coma, dysarthria, dyskinesia, dystonia, euphoria, extrapyramidal syndrome, hypokinesia, increased libido, neuralgia, neuropathy, paranoid ideation, restlessness, suicide attempt, and unmasking tardive dyskinesia.
- Respiratory
- Bronchospasm and pneumonia.
- Skin
- Maculopapular rash, alopecia, angioedema, exfoliative dermatitis, and hirsutism.
- Special Senses
- Accommodation abnormality, dry eye, deafness, increased intraocular pressure, angle-closure glaucoma, and mydriasis.
- Urogenital
- Impotence, polyuria, prostate disorder, abnormal ejaculation, cystitis, dyspareunia, dysuria, gynecomastia, menopause, painful erection, salpingitis, urinary incontinence, urinary retention, and vaginitis.
- OVERDOSAGE
- Human Overdose Experience
- Overdoses of up to 30 grams or more of bupropion have been reported. Seizure was reported in approximately one third of all cases. Other serious reactions reported with overdoses of bupropion alone included hallucinations, loss of consciousness, sinus tachycardia, and ECG changes such as conduction disturbances or arrhythmias. Fever, muscle rigidity, rhabdomyolysis, hypotension, stupor, coma, and respiratory failure have been reported mainly when bupropion was part of multiple drug overdoses.
- Although most patients recovered without sequelae, deaths associated with overdoses of bupropion alone have been reported in patients ingesting large doses of the drug. Multiple uncontrolled seizures, bradycardia, cardiac failure, and cardiac arrest prior to death were reported in these patients.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
0
42912
Other ADRs
74
14117205
Odds Ratio = N/A
Drug Property Information
ATC Code(s):
- A08AA62 - bupropion hydrobromide
- A08AA - Centrally acting antiobesity products
- A08A - "ANTIOBESITY PREPARATIONS, EXCL. DIET PRODUCTS"
- A08 - "ANTIOBESITY PREPARATIONS, EXCL. DIET PRODUCTS"
- A - ALIMENTARY TRACT AND METABOLISM
- N06AX12 - bupropion hydrobromide
- N06AX - Other antidepressants
- N06A - ANTIDEPRESSANTS
- N06 - PSYCHOANALEPTICS
- N - NERVOUS SYSTEM
Active Ingredient:bupropion hydrobromide
Active Ingredient UNII:E70G3G5863
Drugbank ID:DB01156
PubChem Compound:444
CAS Number:34911-55-2
Dosage Form(s):tablet, extended release
Route(s) Of Administrator:oral
Daily Dose:
- 300.0 mg/day N06AX12
Chemical Structure: 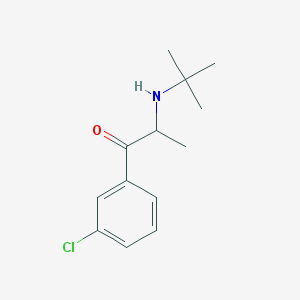

SMILE Code:
CC(C(=O)C1=CC(=CC=C1)Cl)NC(C)(C)C
CC(C(=O)C1=CC(=CC=C1)Cl)NC(C)(C)C
Reference
COHORT STUDY:
N/AOTHER REFERENCE(S):
N/ADisclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.